Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Research Article(ISSN: 2637-6679) 
Factors predicting progression to AML and use of allogeneic hematopoietic stem cell transplant in patients with MDS Volume 7 - Issue 3
Teresa A Simon1*, Gowri Priya Harikrishnan2, H Joachim Deeg3, Tzuyung D Kou1, John H Simon4 and Andres Gomez Caminero1
- 1Worldwide Patient Safety, Bristol Myers Squibb, Princeton, USA
- 2Mu Sigma Inc, Bengaluru, Karnataka, 560066, India
- 3Fred Hutchinson Cancer Research Center, Seattle, USA
- 4Drexel University, Philadelphia, USA
Received: January 22, 2022; Published: January 31, 2022
Corresponding author: Teresa A Simon, is Physicians Research Center, LLC, Toms River, NJ,08755, USA
DOI: 10.32474/RRHOAJ.2018.03.000264
Abstract
Background: Risk-benefit assessment data for myelodysplastic syndrome (MDS) treatment are scarce. We characterized baseline factors and clinical course data in patients with MDS who progressed to acute myeloid leukemia (AML) or underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) in a real-world observational study using demographic, disease, and treatment claims data from a large US administrative healthcare database.
Methods: The study period was from July 1, 2002, to June 30, 2018. The incidence rates (per 1000 patients/year) of progression to AML and undergoing allo-HSCT were evaluated.
Results: The study population comprised 15,680 patients identified using International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification codes for MDS. Mean (range) follow-up duration was 2.8 (0–11.8) and 2.7 (0–11.8) years for the AML and allo-HSCT cohorts, respectively. Risk of progression to AML was high in all age groups, with highest incidence rates in patients aged < 18 years and 50–69 years. Use of azacitidine, indicating higher-risk disease, was associated with both progression to AML and undergoing allo-HSCT. The rate of allo-HSCT was low.
Conclusion: These data emphasize the importance of assessing eligibility to allo-HSCT in a consistent manner.
Keywords: Myelodysplastic syndrome; acute myeloid leukemia; allogeneic hematopoietic stem cell transplantation
Abbreviations: AIDS: Acquired Immune Deficiency Syndrome; Allo-HSCT: allogeneic hematopoietic stem cell; transplantation; AML: acute myeloid leukemia; C: case; CCI: Charlson Comorbidity Index; CI: confidence interval; HCT-CI: Hematopoietic Cell Transplantation-specific Comorbidity Index; HLA: human leukocyte antigen; HR: hazard ratio; ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM: International Classification of Diseases, Tenth Revision, Clinical Modification; IPSS: International Prognostic Scoring System; IPSS-R: International Prognostic Scoring System revised version; IR: incidence rate; MDS: myelodysplastic syndrome; NRM: non-relapse mortality; Optum: Optum® Clinformatics™ Data Mart; SD: standard deviation; SEER; Surveillance, Epidemiology, and End Results
Background
Myelodysplastic syndrome (MDS) represents a heterogeneous group of clonal hematopoietic malignancies associated with progressive bone marrow failure, inadequate hematopoiesis, and cytogenic abnormalities [1-3]. MDS is more common in older patients, with median patient age at diagnosis of 70–75 years [4,5]; the incidence of idiopathic MDS is 4.5/100,000 persons per year in the general population and rises to 26.9–55.4/100,000 persons per year in those aged ≥ 70 years [6]. At the early stage of disease, MDS is mostly asymptomatic and undiagnosed [1]. Over time, patients may experience shortness of breath and fatigue, and develop anemia (refractory to folate and vitamin B12 treatment), neutropenia, and infections as well as thrombocytopenia [1,2]. To assist with treatment decisions, the International Prognostic Scoring System (IPSS) and its revised version (IPSS-R) were developed to assess prognosis in untreated adult patients with idiopathic MDS [7,8]. Depending on the IPSS risk score, median survival times for patients with MDS not treated with chemotherapy are 0.8 (very high risk), 3.0 (intermediate risk), and 8.8 years (very low risk) [7]. Some patients with MDS may experience prolonged survival with a more chronic disease course; however, other patients with a more severe form of MDS may progress to acute myeloid leukemia (AML) [7]. The estimated risk of progression to AML is approximately 3% in the very low risk group and up to 84% in the very high-risk group (risk groups defined per the World Health Organisation PSS) [9]. Historically, the MDS survival data were not collected by the Surveillance, Epidemiology, and End Results (SEER) Program. Reporting of these data by SEER was initiated in 2001.
There is a significant unmet need for safe and effective MDS treatments. Currently, MDS therapy adopts a risk-dependent approach. In patients with higher-risk MDS and clinically relevant cytopenias, the National Comprehensive Cancer Network guidelines recommend hypomethylating agents such as azacitidine and decitabine as first-line treatment; lenalidomide is recommended for use in the subgroup of patients with a 5q chromosome deletion [8]. Long-term myelosuppressive therapy with hypomethylating agents and lenalidomide can present challenges in older patients [10]. Allogeneic hematopoietic stem cell transplantation (allo- HSCT) is the only potentially curative option for MDS. Because of significant morbidity and mortality that may be associated with allo- HSCT, in particular graft-versus-host disease, allo-HSCT tends to be reserved for patients who have good performance status and few co-morbidities [10,11]. The decision to proceed with allo-HSCT is based on disease characteristics, including cytogenetics, patient age, performance status, co-morbid conditions, and psychosocial status, as well as patient preference and availability of a caregiver [12]. Currently, allo-HSCT is primarily offered to patients with higher-risk MDS and to those who progress to AML. However, patients with MDS who progress to AML tend to have a more aggressive disease course, often resulting in a worse performance status and generally poorer health than patients who do not progress; therefore, those who progress to AML are more likely to become unsuitable candidates for allo-HSCT. Allo-HSCT procedure earlier in the disease course has the potential to improve outcomes [12], although the risk of complications such as transplant relapse and graft-versus-host disease must be considered [13]. Prospective trials comparing transplant and non-transplant strategies have recently been completed, but results have not yet been published. Using the medical and pharmacy claims records from a large US administrative healthcare claims database, this study characterized the clinical course of patients with MDS who progressed to AML or underwent allo-HSCT and investigated baseline factors associated with progression to AML or receiving allo-HSCT.
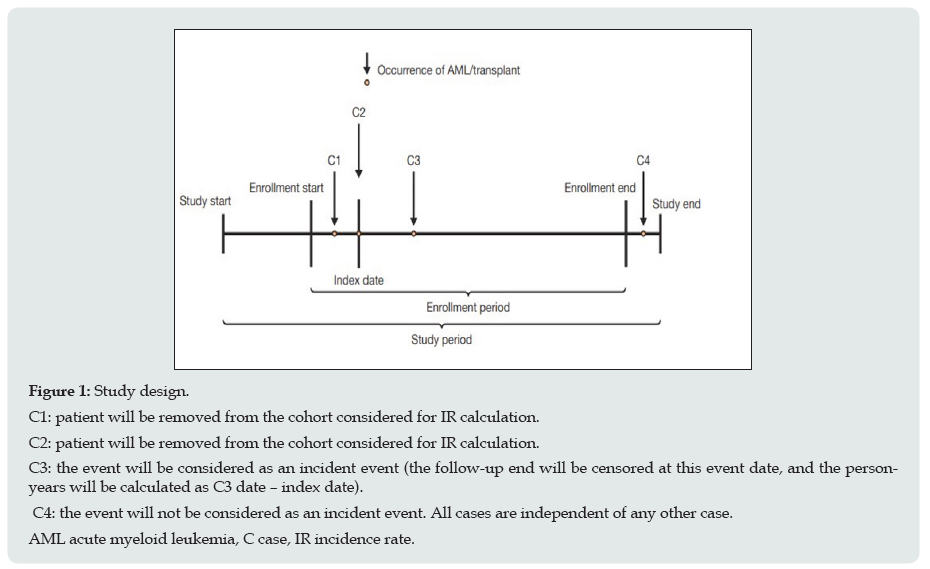
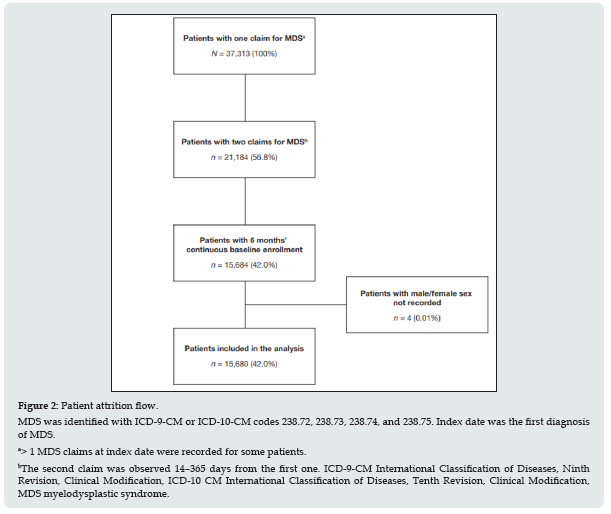
Study design, data sources, and patient population: Data from a large US administrative healthcare database (Optum® Clinformatics™ Data Mart [Optum]) were used for this retrospective, real-world observational study. The Optum database contains claims information and enrollment data on approximately 35 million patients from 2002 onwards. Available data include demographic variables, diagnostic codes, and treatment information. Study design is provided in Figure 1. Patients of any age with a diagnosis of MDS based on the International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM or ICD-10-CM) codes 238.72, 238.73, 238.74, and 238.75 were identified. Patients with at least two claims’ records with a relevant diagnostic code between 14 and 365 days apart were eligible. The index date was defined as the date of the first observed claim record between July 1, 2002, and December 31, 2014; the study period was from July 1, 2002, to June 30, 2018.
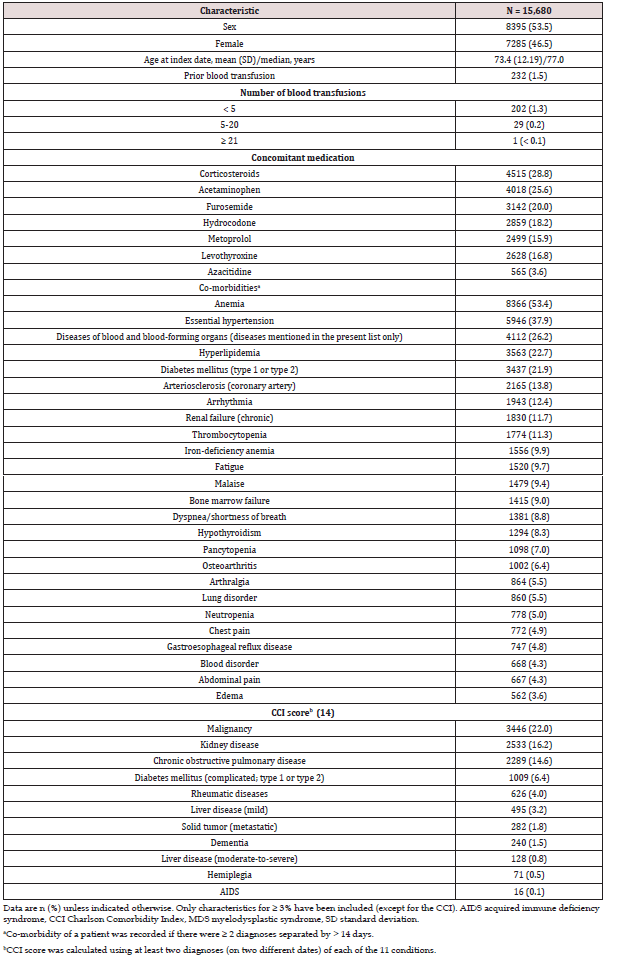
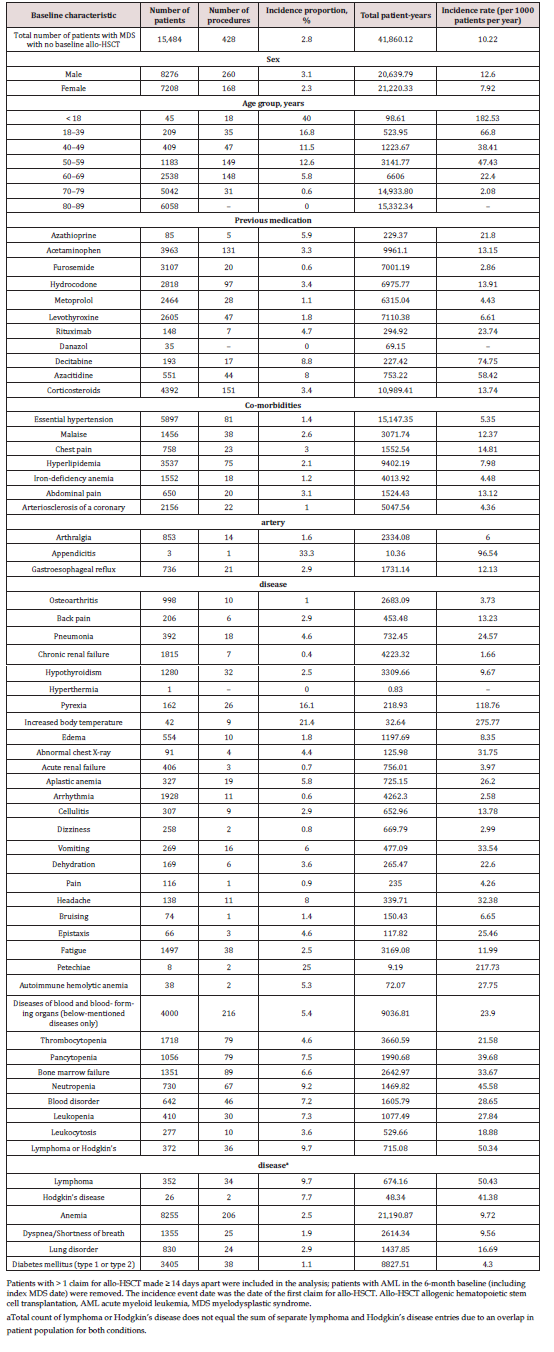
Study variables: Patients who received blood transfusions were identified based on the ICD-9-CM or ICD-10-CM procedure codes in the 6-month baseline period before the index date. Patients with concomitant medication use at the 6-month baseline period were identified based on pharmacy claims records. The co-morbidities of interest were identified among the 50 most frequent co-morbid conditions recorded in the study cohort during the 6-month baseline period for each patient (Table 1). For a co-morbidity to be verified, a confirmation of diagnosis of that co-morbidity was required by at least two records separated by > 14 days. The Charlson Comorbidity Index (CCI) score was calculated to describe the overall burden of disease using a published algorithm on 11 comorbidities in the 6-month baseline period prior to the index date for every eligible patient (Table 2) [14]. The co-morbidities included in the CCI score calculation were dementia, chronic obstructive pulmonary disease, rheumatic diseases, liver disease (mild and moderate-to-severe), complicated diabetes mellitus (type 1 or type 2), hemiplegia, kidney disease, malignancy, metastatic solid tumor, and acquired immunodeficiency syndrome. The Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) scale could not be calculated in this study due to laboratory data being unavailable.
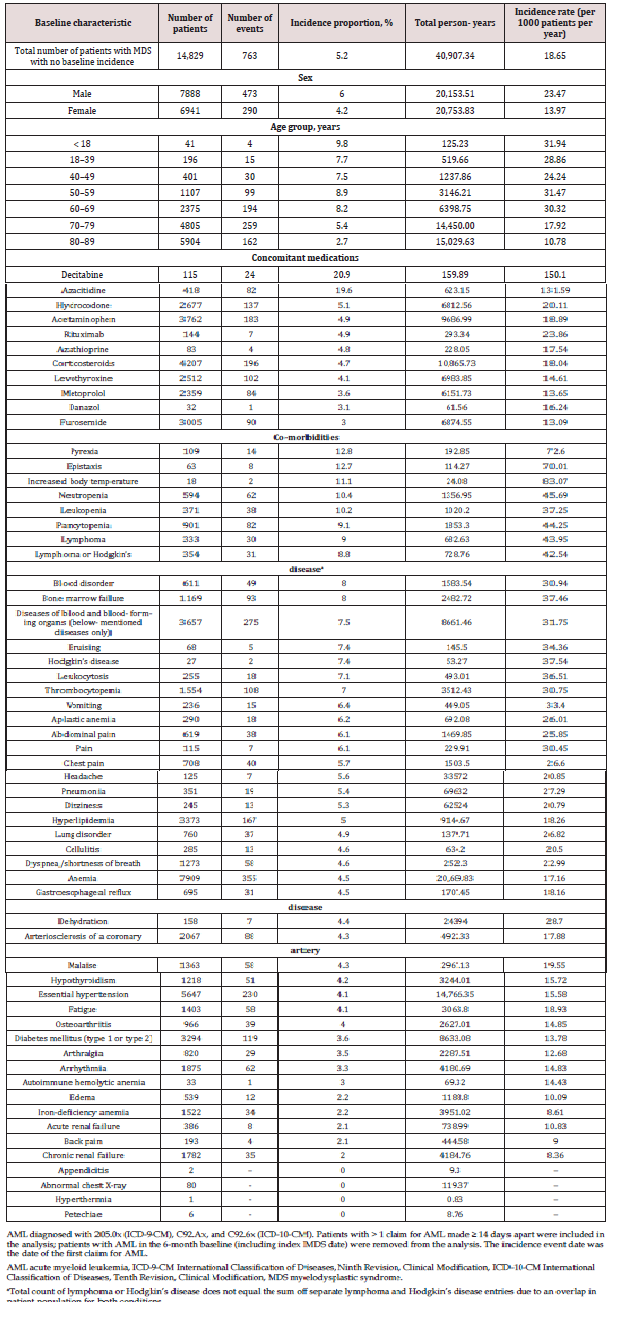
Study outcomes and statistical analyses: Patients with at least two claims that included a relevant ICD-9-CM or ICD-10-CM code for AML (ICD-9-CM: 205.0x; ICD-10-CM: C92.Ax, and C92.6x) or allo-HSCT (ICD-9-CM: V42.4, V42.81, V42.9, 279.50, 996.85; ICD-10-CM: T86.03, Z94.81, Z94.9x, Z94.6x) ≥ 14 days from the index date + 1 day and the study end date were included in the analysis. Baseline characteristics were analyzed descriptively. Only patients without an AML diagnosis or allo-HSCT procedure in the baseline period or at index date were included in the calculation of the incidence rates (IRs) for progression from MDS to AML or undergoing allo-HSCT. IRs were evaluated for the overall cohort and per baseline characteristics (age [groups of < 18, 18–39, 40–49, 50–59, 60–69, 70–79, 80–89 years], sex, concomitant medication, prior procedures, and co-morbidities), and were reported per 1000 patients per year.
To evaluate factors associated with progression to AML and undergoing allo-HSCT, logistic regression modelling was used to identify baseline variables for inclusion in subsequent Cox multivariate models, which were used for covariate selection (at a significance level of 0.05). The list of covariates included age (categories of < 50, 50–69, and 70–89 years), sex, baseline blood transfusions, concomitant medications, and co-morbidities (50 most frequently reported conditions, vide supra). The co-morbidities were included in the model as a categorical variable (presence or absence at baseline) and were excluded using a stepwise method based on the significance assessment. A multivariate Cox regression model was then constructed to determine baseline characteristics associated with occurrence of AML or undergoing allo-HSCT, expressed as adjusted hazard ratios (HRs) with 95% confidence intervals (CIs). The basic assumptions of the models, such as linear relationship between the predictor and the hazard, were observed in the model along with the assumptions of constant hazard rate with time. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient population: 19 of 37,313 patients initially identified under ICD-9-CM and ICD-10-CM codes for MDS, 15,680 were eligible for inclusion and comprised the study population (Figure 2). Mean (range) follow-up duration was 2.8 (0–11.8) and 2.7 (0–11.8) years for the AML and allo-HSCT cohorts, respectively. The minimum of 0-years follow-up duration was attributed to one patient having the first diagnosis of MDS outside of the 6-month baseline period and the second immediately before being diagnosed with AML. At baseline (index date), numbers of male and female patients were comparable. The median patient age was 77.0 years (Table 1). The most common concomitant medications were corticosteroids (28.8%) and acetaminophen (paracetamol; 25.6%). Mean (± standard deviation) CCI score at baseline was 2.99 (± 1.51). The most common co-morbidities were anemia (53.4%; included as a co-morbid condition in this analysis), essential hypertension (37.9%), hyperlipidemia (22.7%), other malignancy (22.0%), and diabetes mellitus (type 1 or type 2, 21.9%).
Incidence and factors associated with progression from MDS to AML: During the study period, 5.2% of patients progressed to AML, representing an IR for progression of 18.7 per 1000 patients per year (Table 2). Progression rates were nearly twice as high in male patients (23.47 per 1000 patients per year) compared with female patients (13.97 per 1000 patients per year). Among age subgroups, the highest progression rates were seen in patients under 18 (31.94 per 1000 patients per year) and between 50 and 59 years of age (31.47 per 1000 patients per year). Patients previously treated with hypomethylating agents at baseline were likely to have the highest incidence of AML, indicating higher-risk disease. Patients younger than 50 years and those aged 50 to 69 years were more likely than patients aged 70–89 to progress to AML (Figure 3a). Receipt of azacitidine, suggesting the presence of higher-risk disease, had the strongest association with progression to AML (HR = 4.55; 95% CI: 3.59–5.76) (Figure 3a). Among comorbidities, epistaxis had the highest association with progression to AML (HR = 3.39; 95% CI: 1.69–6.82); other co-morbidities included pyrexia, lymphoma, pancytopenia, leukopenia, and blood disorders (Figure 3a).
Incidence and factors associated with progression from MDS to undergoing allo-HSCT: During the study period, 2.8% of patients with MDS underwent allo-HSCT, representing a rate of 10.2 per 1000 patients per year (Table 3). Patients aged < 50 years at baseline were more likely to undergo allo-HSCT (HR = 47.59; 95% CI: 31.53–71.84; Figure 3b) compared with older patients. Receipt of azacitidine and decitabine, suggesting the presence of higher-risk disease, were both associated with subsequent allo- HSCT (Figure 3b). Patients who underwent allo-HSCT versus those who did not were more likely to have lymphoma (HR = 2.89; 95% CI: 2.02–4.14), pancytopenia (HR = 2.60; 95% CI: 1.99–3.40), pyrexia (HR = 2.81; 95% CI: 1.83–4.30), leukopenia (HR = 2.28; 95% CI: 1.57–3.32), blood disorder (HR =1.86; 95% CI: 1.37–2.53), or hypothyroidism (HR = 1.85; 95% CI: 1.27–2.68) as a co-morbidity reported at baseline (Figure 3b).
Figure 3: Predictive value of selected baseline variables for MDS progression to AML and advancement to allo-HSCT. Allo-HSCT allogeneic hematopoietic stem cell transplantation, AML acute myeloid leukemia, CI confidence interval, HR hazard ratio, MDS myelodysplastic syndrome.

Discussion
To date, MDS disease course-with its progression to AML or allo- HSCT-has been investigated in several studies with a small sample size. There have been only a few attempts at a population level to characterize clinical characteristics and to calculate the incidence of progression to AML or undergoing allo-HSCT in patients with MDS [7, 15-20]. In this study based on data from a large US-based healthcare claims database, the clinical course of patients with MDS who progressed to AML and of those who underwent allo- HSCT was characterized. Baseline factors associated with potential progression to AML or having an allo-HSCT were also investigated. Anemia (included as a co-morbid condition in this analysis) was among the most common co-morbidities observed at baseline, as expected in patients with MDS. We observed an IR of 18.7 per 1000 patients per year for progression of MDS to AML and two age peaks for the highest progression rates were noted (one in patients aged less than 18 years and another in those aged 50–69 years). It can be hypothesized that the increased IR in patients aged less than 18 years may be attributed to a different biology of the disease in the younger population, with particular germline mutations making these patients predisposed to progression from MDS to AML; however, such an analysis was beyond the scope of this study. The overall incidence rate of patients undergoing allo- HSCT after MDS diagnosis was as low as 10.2 per 1000 patients per year, with patients aged less than 18 years most likely to undergo the procedure. This was an expected finding, considering that allo-HSCT is often reserved for the fittest patients because of potential toxicities [10,12]. Younger patients tend to have a higher performance status and fewer co-morbidities, with lower non- relapse mortality (NRM) after allo-HSCT compared with older individuals. The probability of NRM tends to increase with age, in particular with high intensity (myeloablative) conditioning regimens, highlighting the need for careful assessment of both disease and patient characteristics before initiating allo-HSCT [21]. To date, several predictors of progression to AML in patients with MDS have been identified, including the presence of initiating gene mutations leading to clonal expansion, high percentage of marrow blasts, and infection at referral [22]. In this analysis, treatment with azacitidine was found to be the factor most significantly associated with progression of MDS to AML-an expected finding considering that the presence of higher-risk disease was likely to have been the reason for starting azacitidine. Azacitidine treatment was prominent both in patients with MDS who progressed to AML and in those who underwent allo-HSCT. Interestingly, many patients being treated with azacitidine for evolving AML went on to receive allo-HSCT. Despite being the only potentially curative option for MDS, the rate of allo-HSCT was low in this study. We observed that patients aged < 50 years and those aged 50 to 69 years were more likely to progress to AML than patients aged 70 to 79 years, indicating that age may be an important consideration in how aggressively patients are treated. Our observation reflected the findings from a recent study on prognostic features of secondary AML (defined as AML with an antecedent hematological disease or treatment-related AML), in which AML lacked prognostic value in the elderly patients [15]. Nevertheless, while age itself may not be a clear progression predictor, it is an important consideration when making treatment decisions as it influences other patient-related factors, such as performance status [12]. Currently, allo-HSCT is offered to only a small proportion of patients with MDS as illustrated by the findings of this study. We also observed that patients aged < 50 years were more likely to undergo allo-HSCT versus older patients. However, it has been shown previously that allo-HSCT provides an overall survival benefit in patients aged 60 to 70 years with intermediate- and high-risk MDS [18]. Additionally, allo-HSCT was an effective preventative measure against AML progression [22]. Moreover, an international expert panel recommended that fit patients with high- risk disease by IPSS-R should be considered for allo-HSCT regardless of age; patients with lower-risk disease by IPSS-R and with poor-risk genetic features, profound cytopenias, and high transfusion burden were also suggested as candidates for allo-HSCT [12]. As such, it is important to ensure that the eligibility criteria for patients with MDS considered for allo-HSCT in clinical practice reflect the current evidence as allo-HSCT may provide a valuable, life-saving option for many patients with MDS. There is some encouraging evidence that the number of allo- HSCT procedures is increasing. Based on data from the European Society for Blood and Marrow Transplantation, 940 patients with MDS underwent allo-HSCT in 2004, compared with 2646 in 2015 [23]. This increase is largely attributable to rising numbers of allo- HSCT procedures in older patients (> 60 years); this age cohort accounted for 22% of all transplants in 2004 but increased to 44% in 2015. In addition, there has been an increase in allo-HSCT from human leukocyte antigen (HLA)-matched unrelated donors, from 37% in 2004 to 58% in 2015 [23], and, most recently, in HLA-haploidentical donors [24]. Although determining the type of donors for allo-HSCT was not within the scope of this analysis, we also observed that allo-HSCT was performed in some older patients (> 60 years), but not in patients ≥ 80 years of age in our study. Our finding that patients who underwent allo-HSCT, versus those who did not, were more likely to have lymphoma is consistent with previous descriptions of MDS as a complication of lymphoma treatment [25-27]. Our study has a number of strengths. Firstly, the large sample size obtained from the Optum database allowed for an accurate evaluation of MDS-related claims. In addition, we were able to evaluate patients of all ages, rather than only the elderly ones, which makes the study findings more generalizable. The Optum database was selected as a data source as, in our view, it has a better representation of the Medicare population compared with other databases, such as PharMetrics or MarketScan®. Limitations should also be considered. The retrospective nature of the analysis comes with limitations common to similar studies conducted using administrative claims data. Some information in the claims sources was incomplete, such as duration of treatment with azacitidine and decitabine, and details on allo-HSCT. Also, there was no information available regarding various MDS subtypes or genetics of the disease. Additionally, we believe there is an underestimation of blood transfusions reported in these data: the majority of patients with MDS are generally dependent on blood transfusions and the number of transfusions can be predictive of disease severity and poor prognosis [12,28]. Lastly, the duration of follow-up was short (median: 2.8 years), albeit similar to other published retrospective studies in MDS [29]. Nevertheless, we believe that this study provides valuable further information on the natural trajectory of the disease and factors associated with the decision-making process regarding allo-HSCT.
Conclusion
Overall, this analysis confirmed the heterogeneity of MDS. Baseline characteristics of the patients included in this analysis were investigated for their association with progression to AML and were largely consistent with those from previous studies [30]. With recent advances in our understanding of MDS and the introduction of novel techniques for allo-HSCT, it is important to reassess the eligibility criteria, since more patients than are currently offered the procedure may, in fact, be good candidates. As new medications to treat MDS-associated anemia are becoming available, this analysis could serve as a foundation for future larger studies to include assessment of the effect of new MDS medications on progression to AML or allo-HSCT.
Ethics approval and consent to participate
The study was conducted in accordance with the International Society for Pharmacoepidemiology guidelines for good pharmacoepidemiology practices [31], applicable regulatory requirements, and the 1964 Helsinki Declaration and its later amendments or comaparable ethical standards.
Acknowledgements
The authors are thankful to Marlene Khouri for literature search assistance. Professional medical writing and editorial assistance was provided by Katerina Kumpan, PhD, at Caudex (at the time of writing) and was funded by Bristol Myers Squibb. The authors would like to acknowledge Henry J. Simon, MD. Henry J. Simon, a physician and patient with MDS, was a coauthor of this project in its early stages [32]. Dr. Simon died due to a complication of his MDS: he developed AML in March 2015 and died in July 2015.
References
- Cazzola M, Malcovati L (2005) Myelodysplastic syndromes-coping with ineffective hematopoiesis. N Engl J Med 352(6): 536-538.
- Heaney ML, Golde DW (1999) Myelodysplasia. N Engl J Med 340(21): 1649-1660.
- Cogle CR (2015) Incidence and burden of the myelodysplastic syndromes. Curr Hematol Malig Rep 10(3): 272-281.
- Ma X, Does M, Raza A, Mayne ST (2007) Myelodysplastic syndromes: incidence and survival in the United States. Cancer 109(8): 1536-1542.
- Hofmann WK, Lübbert M, Hoelzer D, Phillip Koeffler H (2004) Myelodysplastic syndromes. Hematol J 5(1): 1-8.
- Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. (2019) SEER Cancer Statistics Review, 1975-2016, National Cancer Institute, Bethesda, MD.
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia Manero G, Solé F, et al. (2012) Revised international prognostic scoring system (IPSS-R) for myelodysplastic syndromes. Blood 120(12): 2454-2465.
- Greenberg PL, Stone RM, Al Kali A, Bejar R, Bennett JM, Brunner AM, et al. (2019) Myelodysplastic syndromes, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15(1): 60-87.
- (2019) American Cancer Society Inc. Survival statistics for myelodysplastic syndromes.
- Klepin HD (2016) Myelodysplastic syndromes and acute myeloid leukemia in the elderly. Clin Geriatr Med 32(1): 155-73.
- Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. (2015) Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 125(8): 1333-1338.
- De Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub Agha I, et al. (2017) Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood 129(13): 1753-1762.
- Bartenstein M, Deeg HJ (2010) Hematopoietic stem cell transplantation for MDS. Hematol Oncol Clin North Am 24(2): 407-422.
- Ternavasio de la Vega H, Castaño Romero F, Ragozzino S, González RS, Vaquero-Herrero M, Siller-Ruiz M, et al. (2018) The updated Charlson Comorbidity Index is a useful predictor of mortality in patients with Staphylococcus aureus bacteraemia. Epidemiol Infect 146(16): 2122-2130.
- Hulegårdh E, Nilsson C, Lazarevic V, Garelius H, Antunovic P, Rangert Derolf Å, et al. (2015) Characterization and prognostic features of secondary acute myeloid leukemia in a population‐based setting: A report from the Swedish Acute Leukemia Registry. Am J Hematol 90(3): 208-214.
- Makishima H, Yoshizato T, Yoshida K, Sekeres MA, Radivoyevitch T, Suzuki H, et al. (2017) Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet 49(2): 204-212.
- Shi J, Shao ZH, Liu H, Bai J, Cao YR, He GS, et al. (2004) Transformation of myelodysplastic syndromes into acute myeloid leukemias. Chin Med J (Engl) 117(7): 963-967.
- Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia Manero G, Malcovati L, et al. (2013) Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol 31(21): 2662-2670.
- Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, et al. (2004) A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 104(2): 579-585.
- Robin M, Porcher R, Ades L, Raffoux E, Michallet M, Francois S, et al. (2015) HLA-matched allogeneic stem cell transplantation improves outcome of higher risk myelodysplastic syndrome A prospective study on behalf of SFGM-TC and GFM. Leukemia 29(7): 1496-1501.
- Danielson N, Byrne M (2020) Indications for allogeneic hematopoietic cell transplantation in myelodysplastic syndrome. Curr Hematol Malig Rep 15(4): 268-275.
- Hospital MA, Vey N (2020) Myelodysplastic syndromes: how to recognize risk and avoid acute myeloid leukemia transformation. Curr Oncol Rep 22(1): 4.
- Robin M, de Witte T (2019) The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. 7th ed: Springer.
- Luznik L, O'Donnell PV, Fuchs EJ (2012) Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol 39(6): 683-693.
- De Roos AJ, Deeg HJ, Davis S (2007) A population-based study of survival in patients with secondary myelodysplastic syndromes (MDS): impact of type and treatment of primary cancers. Cancer Causes Control 18(10): 1199-1208.
- Metayer C, Curtis RE, Vose J, Sobocinski KA, Horowitz MM, Bhatia S, et al. (2003) Myelodysplastic syndrome and acute myeloid leukemia after autotransplantation for lymphoma: a multicenter case-control study. Blood 101(5): 2015-2023.
- Andre M, Mounier N, Leleu X, Sonet A, Brice P, Henry Amar M, et al. (2004) Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: a GELA cohort study on 2837 patients. Blood 103(4): 1222-1228.
- Koutsavlis I (2016) Transfusion thresholds, quality of life, and current approaches in myelodysplastic syndromes. Anemia 2016: 8494738.
- Goldberg SL, Chen E, Corral M, Guo A, Mody Patel N, Pecora AL, et al. (2010) Incidence and clinical complications of myelodysplastic syndromes among United States medicare beneficiaries. J Clin Oncol 28(17): 2847-2852.
- Mutetwa B, Fryzek J, Du Y, Yong M, Sekeres MA, Taioli E (2011) Baseline characteristics and predictors of outcome in patients with myelodysplastic syndromes living in western Pennsylvania. Leuk Lymphoma 52(2): 265-272.
- International Society for Pharmacoepidemiology (2016) Guidelines for Good Pharmacoepidemiology Practices (GPP).
- Simon TA, Kou TD, Gomez A, Simon HJ (2015) Baseline characteristics and the incidence and prevalence of auto-immune hemolytic anemia in myloid dysplastic syndrome (MDS) patients. Leuk Res 39(Suppl 1): S158.

.png)