
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Mini Review(ISSN: 2637-6679) 
Early Decompressive Craniectomy for Post- Thrombolysis Symptomatic Intracranial Haemorrhage Volume 1 - Issue 5
Paul Gunchan1*, Paul Birinder S2 and PL Gautam1
- 1Department of Critical Care Medicine, Dayanand Medical College & Hospital, India
- 2Department of Neurology, Dayanand Medical College & Hospital, India
Received: April 25, 2018; Published: May 02, 2018
Corresponding author: Gunchan Paul, Assistant Professor Critical Care Medicine Dayanand Medical College & Hospita, Ludhiana, Punjab, India
DOI: 10.32474/RRHOAJ.2018.01.000122
Key Message
Intravenous thrombolysis for acute ischemic stroke can be complicated by intracranial haemorrhage. Early decompressive craniectomy in such patients can be life saving but is associated with high risk of peri operative bleeding. We managed such a patient with decompressive craniectomy within 24hrs of thrombolysis by correcting coagulation with the help of thromboelastograhpy.
Keywords: Decompressive craniectomy; Intravenous thrombolysis; Symptomatic intracranial haemorrhage; Thromboelastography
Introduction
Acute ischemic stroke is one of the leading causes of death and permanent disability in the world. Intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (rt-PA) has been the recommended treatment modality in acute ischemic stroke [1]. but the most dreadful complication of thrombolysis is intracerebral haemorrhage in about 7% cases. The clinicians are faced with difficult decision of how to best treat these patients as there are no evidence based guidelines regarding the management of such complications. The American Heart Association has suggested only empirical therapies to replace clotting factors and platelets to reverse coagulopathy [2]. Decompressive craniectomy (DC) is a life-saving procedure for malignant middle cerebral artery stroke associated with cerebral oedema, enough to cause herniation and death [3]. The decision of decompressive craniectomy following intracerebral haemorrhage after intravenous thrombolysis is not without the risk of peri operative haemorrhage. We report the first case where decompressive surgery was uneventfully performed as a life-saving procedure within 24hours of developing symptomatic intracerebral haemorrhage after intravenous thrombolysis. The timing for decompressive craniectomy was guided by thromboelastography (TEG).
Case report
A 63-year old hypertensive, diabetic man presented with left hemiplegia within 140 minutes of onset. On examination, he was alert, GCS 15, left hemiplegia, right gaze palsy and dysarthria, NIHSS (National Institute of Health Stroke Scale) of 17. Magnetic resonance imaging of the brain revealed infarct in the superior division of right middle cerebral artery (MCA) (Figure 1a). His blood biochemistry was unremarkable (Hb-13.8, Plt-145, PT-12.2, and RBS-174). After written consent, thrombolysis was started at 22:10hrs on 11.1.2015 with rt-PA, 5.8mg as bolus followed by 52.7mg infusion over one hour. At 5:30hrs on 12.1.2015, he had upper gastrointestinal bleed followed by impairment in consciousness and his NIHSS score increased to 28. Immediate repeat CT scan of the brain revealed extensive infarction of MCA with haemorrhage in the infarct, extensive oedema and midline shift with uncal herniation (Figure 1b). As he had been recently thrombolysed, his repeat coagulation profile was performed (Hb-10.4, Plt-160, PT-15.2, APTT-27.8, FDP- 256mg/dL) including thromboelastography which was classical of fibrinolysis. Eight units of cryoprecipitate and four units of fresh frozen plasma were transfused in the next six hours and repeat thromboelastography was normal. Then the decision was to proceed with decompressive craniectomy (15:30hr on 12.1.15). A bone window of 12cm in the antero posterior direction in the fronto parieto temporal region was created and duroplasty was performed. The procedure was uneventful. He did not receive any blood products in the peri operative period. Brain CT scan was again performed on the following day and it showed resolution of midline shift with no new hematoma (Figure 1c). He was managed in the intensive care unit with gradual weaning of sedation and ventilation. He was discharged in the sixth week on tracheotomy and NIHSS score of 12. Three months later he was admitted for cranioplasty (Figure 1d) and tracheostomy closure with Mrs Score of 3 (Figure 2a & 2b).
Figure 1: (a) Magnetic resonance imaging of the brain (diffusion weighted image) done at presentation shows acute infarction of the right superior middle cerebral artery. (b) Non contrast CT of the brain done 8 hours after thrombolysis showed haemorrhage in the infarct resulting in mid line shift and mass effect. (c) Non Contrast CT of the brain done on the next day after decompressive craniectomy and hematoma evacuation revealed no new bleed and resolving mass effect.(d) Non Contrast CT of the brain following cranioplasty.
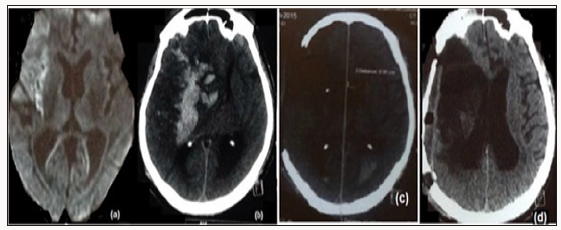
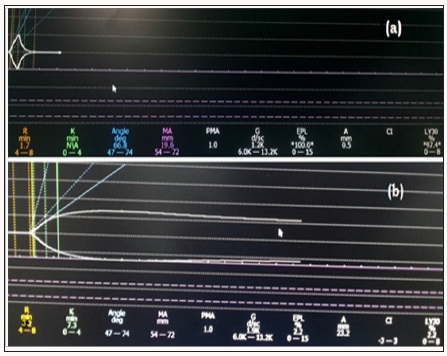
Figure 2: (a) Thromboelastograph trace obtained after 8hr of thrombolysis with R-1.7min, α-66.80, MA-19.6mm, LY30-97.4%, EPL%-100%. These features are characteristic features of fibrinolysis with normal R time, decreased maximum amplitude (MA), raised LY30 (percentage decrease in maximum amplitude or lysis after 30 minutes) and raised EPL. EPL represents the computer prediction of 30mins clot lysis based on interrogation of actual rate of diminution of the trace commencing 30sec post MA with a normal value of <15%. It is the earliest indicator of abnormal lysis. (b) Thromboelastographic trace obtained after infusion of cryoprecipitate and fresh frozen plasma with R-6min, K-1.5min, α-67.50, MA-49.6mm, LY30-0%, EPL%-0%.
Discussion
Thrombolysis remains the treatment of choice in acute ischemic stroke but with increased risk of symptomatic intracranial haemorrhage (ICH).The mortality in these patients is reported to be as high as 45% [4]. There are a few case reports in literature that state DC might be beneficial in the context of post IVT in patients with refractory cerebral oedema [5]. But the most important void is the optimal time to perform DC following thrombolysis. To the best of our knowledge there is only one prior case report where decompressive craniectomy was performed for intracranial haemorrhage following unsuccessful IVT after 48 hour of thrombolysis [6]. Here we report the index case where symptomatic intracranial haemorrhage followed thrombolysis, and was managed by DC and hematoma evacuation within 24 hours of IVT. This early life saving surgery was possible only after rapid correction of coagulation profile with the help of thromboelastography. As, a large series is difficult to be conducted in such cases, it is of interest to report small experiences as ours where the clinical dilemma of performing a surgery following thrombolysis with rt-PA was guided by thromboelastography.
Recombinant t-PA is an exogenous stimulator of the fibrinolytic system that enhances local fibrinolysis by converting plasminogen to plasmin. Our concern was the increased risk of peri operative haemorrhage associated with high mortality due to the persistent effect of TPA. With regard to the pharmacokinetics, half-life of rt- PA is <5 min, with clearance rate of 380-570mL/min [7]. Hence, 80% of rt-PA is cleared from the plasma within 10 minutes of administration. Despite short half-life of rt-PA fibrinolytic effects peak at 4hours and can persist up to 24-48hours [7]. The clinical dilemma in such a scenario was to wait for the disappearance of the fibrinolytic effects to avoid peri operative bleeding at the cost of outweighing the benefits of early DC in reducing the raised ICP. The other option was to efficiently detect and correct the coagulation abnormality by transfusing specific blood products to minimize the risk of bleeding. We had the benefit of thromboelastography at our institute to guide.com with the correction of the deranged coagulation profile before proceeding for DC. S Takeuchi et al. retrospectively reviewed 20 patients who underwent DC for malignant hemispheric infarction after IV TPA administration, with another 20 patients undergoing DC without prior IV TPA administration [8]. They observed intracranial bleeding or worsening of pre existing ICH in two patients (10%) in each group, but tPA was not thought to be contributory to the hemorrhagic events because of the long intervals between the IV tPA and DC(185 and 136h, respectively). However, fibrinolytic markers, such as fibrinogen or fibrin degradation products were unfortunately not measured in the above series.
Thrombelastography or TEG measures the physical properties of the clot via a pin suspended in a cup from a torsion wire connected with a mechanical-electrical transducer. TEG is different from other coagulation tests as it provides global information on the dynamics of clot development, stabilization and dissolution [9]. It assesses both thrombosis and fibrinolysis. Its role is established in cardiac and liver transplant surgery and is being increasingly explored to study role of fibrinolysis in early trauma coagulopathy [10]. Although routinely tested coagulation parameters (BT, CT, PTI, and APTT) were also normal in our case but TEG was characteristic of enhanced fibrinolysis. Hence, we transfused cryoprecipitate and fresh frozen plasma after which the TEG was normal, and we could proceed with surgery.
Conclusion
Decompressive hemicraniectomy with hematoma evacuation following thrombolysis represents an aggressive life saving treatment approach, especially for the patients who develop hemorrhagic complications of intravenous thrombolysis. TEG is one modality which can guide the reversal of deranged coagulation parameter so that major surgery can be undertaken with minimal risk. The decision to proceed with major surgical intervention requires a competent multi disciplinary team as well as an open discussion with relatives as DC may preserve both life and functional ability in well selected patients. More research is needed in this field to elucidate the potential for both modalities in appropriate patients.
References
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, et al. (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359(13): 1317-1329.
- Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, et al. (2007) Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke; Circulation 38(6): 2001-2023.
- Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, et al. (2007) Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke 38(9): 2518-2525.
- Marler JR (1955) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24): 1581-1587.
- Williams A, Sittampalam M, Barua N, Mohd Nor A (2011) Case series of post-thrombolysis patients undergoing hemicraniectomy for malignant anterior circulation ischaemic stroke. Cardiovascular Psychiatry Neurol 2011: 254569.
- Baharvahdat H, Etemadrezaie H, Zabyhian S, Valipour Z, Ganjeifar B, et al. (2014) Decompressive craniectomy after unsuccessful intravenous thrombolysis of malignant cerebral infarction. Iran J Neurol 13: 101-104.
- Tanswell P, Tebbe U, Neuhaus KL, Gläsle Schwarz L, Wojcik J, et al. (1992) Pharmacokinetics and fibrin specificity of alteplase during accelerated infusions in acute myocardial infarction. J Am Coll Cardiol 19(5): 1071- 1105.
- Takeuchi S, Wada K, Nawashiro H, Arimoto H, Ohkawa H et al. (2012) Decompressive craniectomy after intravenous tissue plasminogen activator administration for stroke. Clin Neurol Neurosurg 114(10): 1312-1315.
- Thakur M, Ahmed AB (2012) A review of thromboelastography. International Journal of Perioperative Ultrasound and Applied Technologies 1(1): 25-29.
- Da Luz LT, Nascimento B, Rizoli S (2013) Thromboelastography (TEG®): practical considerations on its clinical use in trauma resuscitation. Scand J Trauma Resusc Emerg Med 2013: 21-29.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

.png)








