
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Research Article(ISSN: 2637-6679) 
Contents of Nineteen Chemical Elements in Thyroid Benign Nodules and Tissue adjacent to Nodules investigated using Neutron Activation Analysis and Inductively Coupled Plasma Atomic Emission Spectrometry Volume 7 - Issue 2
Vladimir Zaichick*
- Department of Radionuclide Diagnostics, Medical Radiological Reseach Centre, Russia
Received: January 03, 2022 Published: January 19, 2022
Corresponding author: V Zaichick, Professor, Department of Radionuclide Diagnostics, Medical Radiological Reseach Centre, Korolyev St. 4, MRRC, Obninsk 249036, Kaluga region, Russia
DOI: 10.32474/RRHOAJ.2022.07.000261
Abstract
Thyroid benign nodules (TBNs) are the most common diseases of this endocrine gland and are common worldwide. The etiology and pathogenesis of TBNs must be considered as multifactorial. The present study was performed to clarify the role of some chemical elements (ChEs) in the etiology of these thyroid disorders. With this aim thyroid tissue levels of aluminum (Al), boron (B), barium (Ba), calcium (Ca), chlorine (Cl), coper (Cu), iron (Fe), I, potassium (K), lithium (Li), magnesium (Mg), manganese (Mn), sodium (Na), phosphorus (P), sulfur (S), silicon (Si), strontium (Sr), vanadium (V), and zinc (Zn) were prospectively evaluated in nodular tissue and tissue adjacent to nodules of 79 patients with TBNs. Measurements were performed using a combination of non-destructive instrumental neutron activation analysis with high resolution spectrometry of short-lived radionuclides and destructive method inductively coupled plasma atomic emission spectrometry. Results of the study were additionally compared with previously obtained data for the same ChEs in “normal” thyroid tissue. It was observed that mass fractions of Al, B, Cl, Cu, Fe, Li, Mn, Na, P, S, Si, V, and Zn contents in “nodular” tissue were higher, while Ca and I content was lower in comparison with contents of these ChEs in normal gland. Mass fractions of Cl and Na in “adjacent” group of samples were approximately 2.7 and 1.6 times, respectively, higher than in “normal” thyroid. Contents of Al, B, Ba, Br, Ca, Cl, Cu, K, Mg, Mn, Na, P, S, Sr, V, and Zn found in the “nodular” and “adjacent” groups of thyroid tissue samples were very similar, however, mass fraction of Fe was lower, while mass fraction of I was higher in “adjacent” group of samples. At that, level of I in “adjacent” group of samples was over 2 times higher than in nodular tissue and almost equals the normal value. Finally, this study provides evidence on many ChEs level alteration in nodular and adjacent to nodule tissue and shows the necessity to continue ChEs research of TBNs. The little reduced level of I content in nodular tissue could possibly be explored for differential diagnosis of TBNs and thyroid cancer.
Keywords: Thyroid; Thyroid benign nodules; Chemical elements; Neutron activation analysis; Inductively coupled plasma atomic emission spectrometry
Introduction
Thyroid benign nodules (TBNs) are universally encountered
and frequently detected by palpation during a physical examination,
or incidentally, during clinical imaging procedures. TBNs include
non-neoplastic lesions, for example, colloid goiter and thyroiditis,
as well as neoplastic lesions such as thyroid adenomas [1-3]. For
over 20th century, there was the dominant opinion that TBNs is the
simple consequence of iodine deficiency. However, it was found
that TBNs is a frequent disease even in those countries and regions
where the population is never exposed to iodine shortage [4].
Moreover, it was shown that iodine excess has severe consequences
on human health and associated with the presence of TBNs [5-
8]. It was also demonstrated that besides the iodine deficiency
and excess many other dietary, environmental, and occupational
factors are associated with the TBNs incidence [9-11]. Among these
factors a disturbance of evolutionary stable input of many chemical
elements (ChEs) in human body after industrial revolution plays a
significant role in etiology of TBNs [12].
Besides iodine, many other ChEs have also essential
physiological functions [13]. Essential or toxic (goitrogenic,
mutagenic, carcinogenic) properties of ChEs depend on tissuespecific
need or tolerance, respectively [13]. Excessive accumulation
or an imbalance of the ChEs may disturb the cell functions and
may result in cellular proliferation, degeneration, death, benign
or malignant transformation [13-15]. In our previous studies
the complex of in vivo and in vitro nuclear analytical and related
methods was developed and used for the investigation of iodine and
other ChEs contents in the normal and pathological thyroid [16-
22]. Iodine level in the normal thyroid was investigated in relation
to age, gender and some non-thyroidal diseases [23,24]. After
that, variations of many ChEs content with age in the thyroid of
males and females were studied and age- and gender-dependence
of some ChEs was observed [25-41]. Furthermore, a significant
difference between some TEs contents in colloid goiter, thyroiditis,
and thyroid adenoma in comparison with normal thyroid was
demonstrated [42-45].
To date, the etiology and pathogenesis of TBNs must be
considered as multifactorial. The present study was performed to
find out differences in ChEs contents between the group of nodular
tissues and tissue adjacent to nodules, as well as to clarify the role
of some ChEs in the etiology of TBNs. Having this in mind, the
aim of this exploratory study was to examine differences in the
content of aluminum (Al), boron (B), barium (Ba), calcium (Ca),
chlorine (Cl), coper (Cu), iron (Fe), I, potassium (K), lithium (Li),
magnesium (Mg), manganese (Mn), sodium (Na), phosphorus (P),
sulfur (S), silicon (Si), strontium (Sr), vanadium (V), and zinc (Zn)
in nodular and adjacent to nodules tissues of thyroids with TBNs
using a combination of non-destructive instrumental neutron
activation analysis with high resolution spectrometry of shortlived
radionuclides (INAA-SLR) and destructive method such as
inductively coupled plasma atomic emission spectrometry (ICPAES),
and to compare the levels of these ChEs in two groups (nodular
and adjacent to nodules tissues) of the cohort of TBNs samples.
Moreover, for understanding a possible role of ChEs in etiology
and pathogenesis of TBNs results of the study were compared with
previously obtained data for the same ChEs in “normal” thyroid
tissue [42-45].
Material and Methods
All 79 patients suffered from TBNs (46 patients with colloid
goiter, mean age M+SD was 48+12 years, range 30-64; 19 patients
with thyroid adenoma, mean age M+SD was 41+11 years, range
22-55; and 14 patients with thyroiditis, mean age M+SD was
39+9 years, range 34-50) were hospitalized in the Head and Neck
Department of the Medical Radiological Research Centre (MRRC),
Obninsk. The group of patients with thyroiditis included 8 persons
with Hashimoto’s thyroiditis and 6 persons with Riedel’s Struma.
Thick-needle puncture biopsy of suspicious nodules of the thyroid
was performed for every patient, to permit morphological study of
thyroid tissue at these sites and to estimate their ChEs contents.
For all patients the diagnosis has been confirmed by clinical and
morphological/histological results obtained during studies of
biopsy and resected materials. “Normal” thyroids for the control
group samples were removed at necropsy from 105 deceased
(mean age 44+21 years, range 2-87), who had died suddenly. The
majority of deaths were due to trauma. A histological examination
in the control group was used to control the age norm conformity, as
well as to confirm the absence of micro-nodules and latent cancer.
All studies were approved by the Ethical Committees of
MRRC. All the procedures performed in studies involving human
participants were in accordance with the ethical standards of
the institutional and/or national research committee and with
the 1964 Helsinki declaration and its later amendments, or with
comparable ethical standards. Informed consent was obtained
from all individual participants included in the study. All tissue
samples were divided into two portions using a titanium scalpel
[46]. One was used for morphological study while the other was
intended for ChEs analysis. After the samples intended for ChEs
analysis were weighed, they were freeze-dried and homogenized
[47]. The pounded samples weighing about 10 mg (for biopsy) and
100 mg (for resected materials) were used for ChE measurement
by INAA-SLR. The content of Ca, Cl, I, K, Mg, Mn, and Na were
determined by INAA-SLR using a horizontal channel equipped with
the pneumatic rabbit system of the WWR-c research nuclear reactor
(Branch of Karpov Institute, Obninsk). After non-destructive INAASLR
investigation the thyroid samples were used for ICP-AES. The
samples were decomposed in autoclaves and aliquots of solutions
were used to determine the Al, B, Ba, Ca, Cu, Fe, K, Li, Mg, Mn, Na, P,
S, Si, Sr, V, and Zn mass fractions by ICP-AES using the Spectrometer
ICAP-61 (Thermo Jarrell Ash, USA). Information detailing with
the NAA-SLR and ICP-AES methods used and other details of the
analysis were presented in our earlier publications concerning ChE
contents in human thyroid [33,34], prostate [48-52], and scalp hair
[53].
To determine contents of the ChE by comparison with a known
standard, biological synthetic standards (BSS) prepared from
phenol-formaldehyde resins were used [54]. In addition to BSS,
aliquots of commercial, chemically pure compounds were also used
as standards. Ten sub-samples of certified reference material (CRM)
IAEA H-4 (animal muscle) and five sub-samples of CRM of the
Institute of Nuclear Chemistry and Technology (INCT, Warszawa,
Poland) INCT-SBF-4 Soya Bean Flour, INCT-TL-1 Tea Leaves, and
INCT-MPH-2 Mixed Polish Herbs were treated and analyzed in the
same conditions that thyroid samples to estimate the precision
and accuracy of results A dedicated computer program for INAASLR
mode optimization was used [55]. All thyroid samples for
ChEs analysis were prepared in duplicate and mean values of
ChEs contents were used in final calculation. Mean values of ChE
contents were used in final calculation for the Ca, K, Mg, Mn, and Na
mass fractions measured by two methods. Using Microsoft Office
Excel software, a summary of the statistics, including, arithmetic
mean, standard deviation, standard error of mean, minimum and
maximum values, median, percentiles with 0.025 and 0.975 levels
was calculated for ChEs contents in nodular and adjacent tissue of
thyroids with TBNs. Data for “normal” thyroid were taken from our previous publications [42-45]. The difference in the results between
three groups of samples (“normal”, “nodular”, and “adjacent”) was
evaluated by the parametric Student’s t-test and non-parametric
Wilcoxon-Mann-Whitney U-test.
Results
Table 1 presents certain statistical parameters (arithmetic mean, standard deviation, standard error of mean, minimal and maximal values, median, percentiles with 0.025 and 0.975 levels) of the Al, B, Ba, Ca, Cl, Cu, Fe, I, K, Li, Mg, Mn, Na, P, S, Si, Sr, V, and Zn mass fraction in nodular and adjacent to nodules tissue of thyroid with TBN (“nodular” and “adjacent” group of thyroid tissue samples). The ratios of means and the comparison of mean values of Al, B, Ba, Ca, Cl, Cu, Fe, I, K, Li, Mg, Mn, Na, P, S, Si, Sr, V, and Zn mass fractions in pairs of sample groups such as “normal” and “nodular”, “normal” and “adjacent”, and also “adjacent” and “nodular” are presented in Tables 2, 3 & 4, respectively.
Table 1: Some statistical parameters of Al, B, Ba, Ca, Cl, Cu, Fe, I, K, Li, Mg, Mn, Na, P, S, Si, Sr, V, and Zn mass fraction (mg/kg, dry mass basis) in (nodular and adjacent tissue of thyroid benign nodules (TBN)
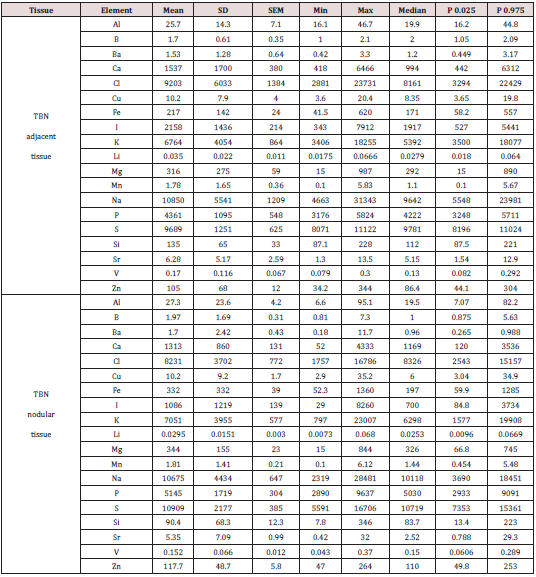
M - arithmetic mean, SD - standard deviation, SEM - standard error of mean, Min - minimum value, Max -maximum value, P 0.025 -percentile with 0.025 level, P 0.975 - percentile with 0.975 level.
Table 2: Differences between mean values (M+SEM) of Al, B, Ba, Ca, Cl, Cu, Fe, I, K, Li, Mg, Mn, Na, P, S, Si, Sr, V, and Zn mass fraction (mg/kg, dry mass basis)) in normal thyroid (NT) and thyroid benign nodules (TBN) (nodular tissue).
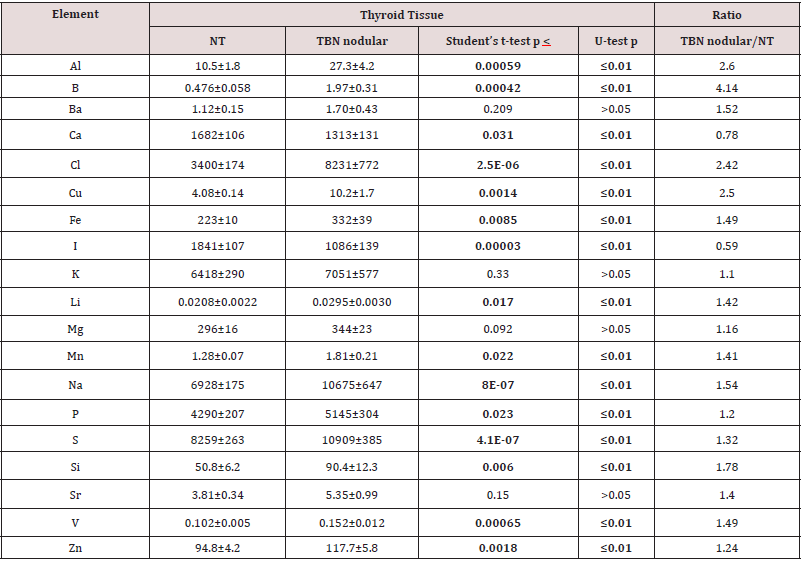
M – arithmetic mean, SEM – standard error of mean, Statistically significant values are in bold
Table 3: Differences between mean values (M+SEM) of Al, B, Ba, Ca, Cl, Cu, Fe, I, K, Li, Mg, Mn, Na, P, S, Si, Sr, V, and Zn mass fraction (mg/kg, dry mass basis) ) in normal thyroid (NT) and thyroid tissue adjacent to benign nodules (TBN adjacent)
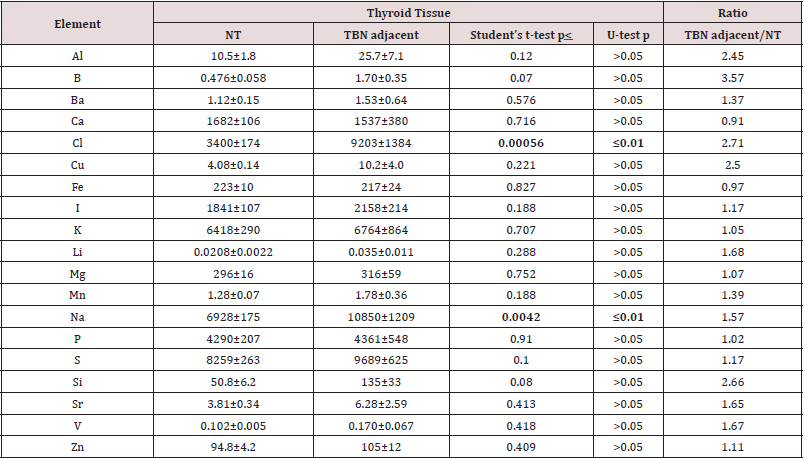
M - arithmetic mean, SEM - standard error of mean, statistically significant values are in bold.
Table 4: Differences between mean values (M+SEM) of Al, B, Ba, Ca, Cl, Cu, Fe, I, K, Li, Mg, Mn, Na, P, S, Si, Sr, V, and Zn mass fraction (mg/kg, dry mass basis) in nodular (TBN nodular) and thyroid tissue adjacent to benign nodules (TBN adjacent).
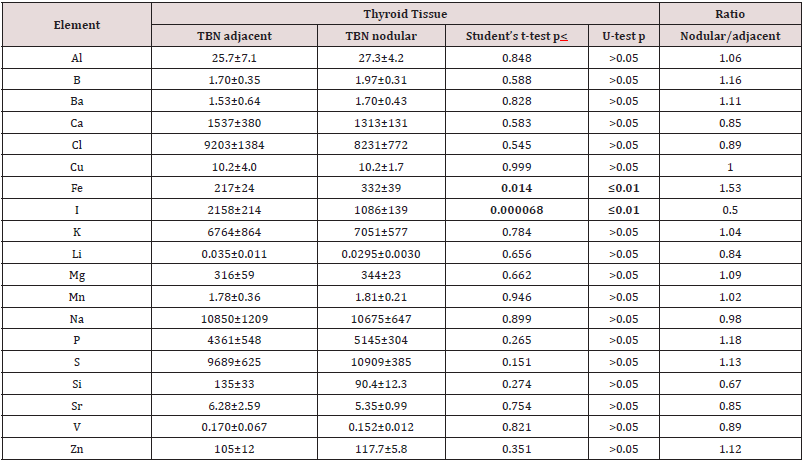
M - arithmetic mean, SEM - standard error of mean, statistically significant values are in bold.
Discussion
As was shown before [33,34,48-53] good agreement of the Al, B, Ba, Br, Ca, Cl, Cu, Fe, I, K, Mg, Mn, Na, P, S, Sr, V, and Zn mass fractions in CRM IAEA H-4, INCT-SBF-4, INCT-TL-1, and INCT-MPH-2 samples determined by both INAA-SLR and ICP-AES methods with the certified data of these CRMs indicates acceptable accuracy of the results obtained in the study of thyroid tissue samples presented in Tables 1–4. The Al, B, Cl, Cu, Fe, Li, Mn, Na, P, S, Si, V, and Zn contents in “nodular” tissue were higher, while Ca and I content was lower in comparison with contents of these ChEs in normal gland (Table 2). Significant differences between ChEs contents of “normal” thyroid and ChEs contents of thyroid tissue adjacent to nodules were found for Cl and Na. Mass fractions of Cl and Na in “adjacent” group of samples were approximately 2.7, and 1.6 times, respectively, higher than in “normal” thyroid (Table 3). In a general sense Al, B, Ba, Br, Ca, Cl, Cu, K, Mg, Mn, Na, P, S, Sr, V, and Zn contents found in the “nodular” and “adjacent” groups of thyroid tissue samples were very similar (Table 4). However, mass fraction of Fe was lower, while mass fraction of I was higher in “adjacent” group of samples (Table 4). At that, level of I in “adjacent” group of samples was over 2 times higher than in nodular tissue (Table 4) and almost equals the normal value (Table 3).
Characteristically, elevated or reduced levels of ChEs observed in thyroid nodules are discussed in terms of their potential role in the initiation and promotion of these thyroid lesions. In other words, using the low or high levels of the ChEs in affected thyroid tissues researchers try to determine the role of the deficiency or excess of each ChEs in the etiology and pathogenesis of thyroid diseases. In our opinion, abnormal levels of many ChEs in TBNs could be and cause, and also effect of thyroid tissue transformation. From the results of such kind studies, it is not always possible to decide whether the measured decrease or increase in ChEs level in pathologically altered tissue is the reason for alterations or vice versa. According to our opinion, investigation of ChEs contents in thyroid tissue adjacent to nodules and comparison obtained results with ChEs levels typical of “normal” thyroid gland may give additional useful information on the topic because this data show conditions of tissue in which TBNs were originated and developed. For example, results of this study demonstrated that contents of Cl and Na in thyroid “adjacent” tissue in which TBNs were originated and developed were significantly higher the levels which are “normal” for thyroid gland, while content of I was “normal”. In turn, in nodular tissue content of Fe was significantly higher, whereas content of I was almost 2 times lower than in tissues adjacent to nodules.
Chlorine and sodium
Cl and Na are ubiquitous, extracellular electrolytes essential to more than one metabolic pathway. In the body, Cl and Na mostly present as sodium chloride. Therefore, as usual, there is a correlation between Na and Cl contents in tissues and fluids of human body. Because Cl is halogen like I, in the thyroid gland the biological behavior of chloride has to be similar to the biological behavior of iodide. The main source of natural Cl for human body is salt in food and chlorinated drinking water. Environment (air, water and food) polluted by artificial nonorganic Cl-contained compounds, for example such as sodium chlorate (NaClO3), and organic Cl-contained compounds, for example such as polychlorinated biphenyls (PCBs) and dioxin, is other source. There is a clear association between using chlorinated drinking water, levels NaClO3, PCBs and dioxin in environment and thyroid disorders, including cancer [56-60]. Thus, on the one hand, the accumulated data suggest that Cl level in thyroid tissue might be responsible for TBNs development. However, on the other hand, it is well known that Cl and Na mass fractions in human tissue samples depend mainly on the extracellular water volume [61]. Nodular and adjacent to nodules thyroid tissues can be more vascularized and can contain more relative volume of colloid that normal thyroid. Because blood and colloid are extracellular liquids, it is possible to speculate that it could be the reason for elevated levels of Cl and Na in TBNs and adjacent tissue. If that is the case the equilibrium between Cl and Na increases has to be, however, in comparison with “normal” thyroid the change of Cl level in TBNs and adjacent tissue is significantly higher than change of Na level. Thus, it is possible to assume that an excessive accumulation of Cl in thyroid tissue is involved in TBNs etiology. Overall, the elevated levels of Cl in thyroid tissue could possibly be explored as risk factor of TBNs.
Iron
It is well known that Fe as TEs is involved in many very important functions and biochemical reactions of human body. Fe metabolism is therefore very carefully regulated at both a systemic and cellular level [62,63]. Under the impact of age and multiple environmental factors the Fe metabolism may become dysregulated with attendant accumulation of this metal excess in tissues and organs, including thyroid [25,26,29-35]. Most experimental and epidemiological data support the hypothesis that Fe overload is a risk factor for benign and malignant tumors [64]. This goitrogenic and oncogenic effect could be explained by an overproduction of ROS and free radicals [65]. Thus, on the one hand, the accumulated data suggest that Fe might be responsible for TBNs development. But, on the other hand, the elevated level of Fe was not found in thyroid tissue adjacent to nodules. It is well known that blood is the main pool for Fe in human body and therefore high vascularisation of nodular tissue may be the reason for Fe elevated levels in TBNs [66].
Iodine
To date, it was well established that I deficiency or excess has severe consequences on human health and associated with the presence of TBNs [5-8,67]. However, in present study neither reduced nor elevated levels of I in thyroid tissue adjacent to nodules in comparison with “normal” thyroid tissue were not found. Compared to other soft tissues, the human thyroid gland has higher levels of I, because this element plays an important role in its normal functions, through the production of thyroid hormones (thyroxin and triiodothyronine) which are essential for cellular oxidation, growth, reproduction, and the activity of the central and autonomic nervous system. As was shown in present study, benign nodular transformation is probably accompanied by a partial loss of tissue-specific functional features, which leads to a modest reduction in I content associated with functional characteristics of the human thyroid tissue. Little reduced level of I content in nodular tissue could possibly be explored for differential diagnosis of TBNs and thyroid cancer, because, as was found in our ealier studies, thyroid malignant trasformation is accompanied by a drastically loss of I accumulation [18, 68-70].
Limitations
This study has several limitations. Firstly, analytical techniques employed in this study measure only nineteen ChE (Al, B, Ba, Ca, Cl, Cu, Fe, I, K, Li, Mg, Mn, Na, P, S, Si, Sr, V, and Zn) mass fractions. Future studies should be directed toward using other analytical methods which will extend the list of ChEs investigated in “normal” thyroid and in pathologically altered tissue. Secondly, the sample size of TBNs group was relatively small and prevented investigations of ChEs contents in this group using differentials like gender, histological types of TBNs, nodules functional activity, stage of disease, and dietary habits of patients with TBNs. Lastly, generalization of our results may be limited to Russian population. Despite these limitations, this study provides evidence on many ChEs level alteration in nodular and adjacent to nodule tissue and shows the necessity to continue ChEs research of TBNs.
Conclusion
In this work, ChEs analysis was carried out in the tissue samples of TBNs using a combination of non-destructive INAA-SLR and destructive ICP-AES methods. It was shown that this combination is an adequate analytical tool for the determination of Al, B, Ba, Ca, Cl, Cu, Fe, I, K, Li, Mg, Mn, Na, P, S, Si, Sr, V, and Zn content in the tissue samples of human thyroid in norm and pathology, including needle-biopsy specimens. It was observed that mass fractions of Al, B, Cl, Cu, Fe, Li, Mn, Na, P, S, Si, V, and Zn contents in “nodular” tissue were higher, while Ca and I content was lower in comparison with contents of these ChEs in normal gland. Mass fractions of Cl and Na in “adjacent” group of samples were approximately 2.7 and 1.6 times, respectively, higher than in “normal” thyroid. Contents of Al, B, Ba, Br, Ca, Cl, Cu, K, Mg, Mn, Na, P, S, Sr, V, and Zn found in the “nodular” and “adjacent” groups of thyroid tissue samples were very similar, however, mass fraction of Fe was lower, while mass fraction of I was higher in “adjacent” group of samples. At that, level of I in “adjacent” group of samples was over 2 times higher than in nodular tissue and almost equals the normal value.
Acknowledgements
The author is extremely grateful to Profs. Vtyurin BM and Medvedev VS, Medical Radiological Research Center, Obninsk, as well as to Dr. Choporov Yu, former Head of the Forensic Medicine Department of City Hospital, Obninsk, for supplying thyroid samples. The author is also grateful to Dr. Karandaschev V, Dr. Nosenko S, and Moskvina I, Institute of Microelectronics Technology and High Purity Materials, Chernogolovka, Russia, for their help in ICP-AES analysis.
References
- Ghartimagar D, Ghosh A, Shrestha MK, Thapa S, Talwar OP (2020) Histopathological Spectrum of Non-Neoplastic and Neoplastic Lesions of Thyroid: A Descriptive Cross-sectional Study. J Nepal Med Assoc 58(231): 856-861.
- Hoang VT, Trinh CT (2020) A Review of the Pathology, Diagnosis and Management of Colloid Goitre. Eur Endocrinol 16(2): 131-135.
- Popoveniuc G, Jonklaas J (2012) Thyroid nodules. Med Clin North Am 96(2): 329-349.
- Derwahl M, Studer H (2001) Multinodular goitre: 'much more to it than simply iodine deficiency'. Baillieres Best Pract Res Clin Endocrinol Metab 14(4): 577-600.
- Zaichick V (1998) Iodine excess and thyroid cancer. J Trace Elem Exp Med 11(4): 508-509.
- Zaichick V, Iljina T (1998) Dietary iodine supplementation effect on the rat thyroid 131I blastomogenic action. In: Die Bedentung der Mengen- und Spurenelemente. 18. Arbeitstangung. Jena: Friedrich-Schiller-Universität, 1998: 294-306.
- Kim S, Kwon YS, Kim JY, Hong KH, Park YK (2019) Association between iodine nutrition status and thyroid disease-related hormone in Korean adults: Korean National Health and Nutrition Examination Survey VI (2013-2015). Nutrients 11(11): 2757.
- Vargas Uricoechea Р, Pinzón Fernández MV, Bastidas Sánchez BE, Jojoa Tobar E, Ramírez Bejarano LE, et al. (2019) Iodine status in the colombian population and the impact of universal salt iodization: a double-edged sword? J Nutr Metab 2019: 6239243.
- Stojsavljević A, Rovčanin B, Krstić D, Borković Mitić S, Paunović I, et al. (2019) Risk assessment of toxic and essential trace metals on the thyroid health at the tissue level: The significance of lead and selenium for colloid goiter disease. Expo Health.
- Fahim YA, Sharaf NE, Hasani IW, Ragab EA, Abdelhakim HK (2020) Assessment of thyroid function and oxidative stress state in foundry workers exposed to lead. J Health Pollut 10(27): pp.200903.
- Liu M, Song J, Jiang Y, Lin Y, Peng J, et al. (2021) A case-control study on the association of mineral elements exposure and thyroid tumor and goiter. Ecotoxicol Environ Saf 208: pp.111615.
- Zaichick V (2006) Medical elementology as a new scientific discipline. J Radioanal Nucl Chem 269: 303-309.
- Moncayo R, Moncayo H (2017) A post-publication analysis of the idealized upper reference value of 2.5 mIU/L for TSH: Time to support the thyroid axis with magnesium and iron especially in the setting of reproduction medicine. BBA Clin 7: 115-119.
- Beyersmann D, Hartwig A (2008) Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol 82(8): 493-512.
- Martinez Zamudio R, Ha HC (2011) Environmental epigenetics in metal exposure. Epigenetics 6(7): 820-827.
- Zaĭchik VE, Raibukhin YuS, Melnik AD, Cherkashin VI (1970) Neutron-activation analysis in the study of the behavior of iodine in the organism. Med Radiol (Mosk) 15(1): 33-36.
- Zaĭchik VE, Matveenko EG, Vtiurin BM, Medvedev VS (1982) Intrathyroid iodine in the diagnosis of thyroid cancer. Vopr Onkol 28(3): 18-24.
- Zaichick V, Tsyb AF, Vtyurin BM (1995) Trace elements and thyroid cancer. Analyst 120(3): 817-821.
- Zaichick VYe, Choporov YuYa (1996) Determination of the natural level of human intra-thyroid iodine by instrumental neutron activation analysis. J Radioanal Nucl Chem 207(1): 153-161.
- Zaichick V (1998) In vivo and in vitro application of energy dispersive XRF in clinical investigations: experience and the future. J Trace Elem Exp Med 11(4): 509-510.
- Zaichick V, Zaichick S (1999) Energy-dispersive X-ray fluorescence of iodine in thyroid puncture biopsy specimens. J Trace Microprobe Tech 17(2): 219-232.
- Zaichick V (2000) Relevance of, and potentiality for in vivo intrathyroidal iodine determination. Ann N Y Acad Sci 904: 630-632.
- Zaichick V, Zaichick S (1997) Normal human intrathyroidal iodine. Sci Total Environ 206(1): 39-56.
- Zaichick V (1999) Human intrathyroidal iodine in health and non-thyroidal disease. In: New aspects of trace element research (Eds: M.Abdulla, M.Bost, S.Gamon, P.Arnaud, G.Chazot). London, Smith-Gordon, and Tokyo: Nishimura 1999: 114-119.
- Zaichick V, Zaichick S (2017) Age-related changes of some trace element contents in intact thyroid of females investigated by energy dispersive X-ray fluorescent analysis. Trends Geriatr Healthc 1(1): 31-38.
- Zaichick V, Zaichick S (2017) Age-related changes of some trace element contents in intact thyroid of males investigated by energy dispersive X-ray fluorescent analysis. MOJ Gerontol Ger 1(5): pp.00028.
- Zaichick V, Zaichick S (2017) Age-related changes of Br, Ca, Cl, I, K, Mg, Mn, and Na contents in intact thyroid of females investigated by neutron activation analysis. Curr Updates Aging 1: p.51.
- Zaichick V, Zaichick S (2017) Age-related changes of Br, Ca, Cl, I, K, Mg, Mn, and Na contents in intact thyroid of males investigated by neutron activation analysis. J Aging Age Relat Dis 1(1): pp.1002.
- Zaichick V, Zaichick S (2017) Age-related changes of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn contents in intact thyroid of females investigated by neutron activation analysis. J Gerontol Geriatr Med 3: pp. 015.
- Zaichick V, Zaichick S (2017) Age-related changes of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn contents in intact thyroid of males investigated by neutron activation analysis. Curr Trends Biomedical Eng Biosci 4(4): 555644.
- Zaichick V, Zaichick S (2018) Effect of age on chemical element contents in female thyroid investigated by some nuclear analytical methods. MicroMedicine 6(1): 47-61.
- Zaichick V, Zaichick S (2018) Neutron activation and X-ray fluorescent analysis in study of association between age and chemical element contents in thyroid of males. Op Acc J Bio Eng Bio Sci 2(4): 202-212.
- Zaichick V, Zaichick S (2018) Variation with age of chemical element contents in females’ thyroids investigated by neutron activation analysis and inductively coupled plasma atomic emission spectrometry. J Biochem Analyt Stud 3(1): 1-10.
- Zaichick V, Zaichick S (2018) Association between age and twenty chemical element contents in intact thyroid of males. SM Gerontol Geriatr Res 2(1):1014.
- Zaichick V, Zaichick S (2018) Associations between age and 50 trace element contents and relationships in intact thyroid of males. Aging Clin Exp Res 30(9):1059–1070.
- Zaichick V, Zaichick S (2018) Possible role of inadequate quantities of intra-thyroidal bromine, rubidium and zinc in the etiology of female subclinical hypothyroidism. EC Gynaecology 7(3):107-115.
- Zaichick V, Zaichick S (2018) Possible role of inadequate quantities of intra-thyroidal bromine, calcium and magnesium in the etiology of female subclinical hypothyroidism. Int Gyn and Women’s Health 1(3).
- Zaichick V, Zaichick S (2018) Possible role of inadequate quantities of intra-thyroidal cobalt, rubidium and zinc in the etiology of female subclinical hypothyroidism. Womens Health Sci J 2(1):000108.
- Zaichick V, Zaichick S (2018) Association between female subclinical hypothyroidism and inadequate quantities of some intra-thyroidal chemical elements investigated by X-ray fluorescence and neutron activation analysis. Gynaecology and Perinatology 2(4): 340-355.
- Zaichick V, Zaichick S (2018) Investigation of association between the high risk of female subclinical hypothyroidism and inadequate quantities of twenty intra-thyroidal chemical elements. Clin Res: Gynecol Obstet 1(1):1-18.
- Zaichick V, Zaichick S (2018) Investigation of association between the high risk of female subclinical hypothyroidism and inadequate quantities of intra-thyroidal trace elements using neutron activation and inductively coupled plasma mass spectrometry. Acta Scientific Medical Sciences 2(9): 23-37.
- Zaichick V (2021) Determination of Twenty Chemical Element Contents in Macro- and Micro-Follicular Colloid Goiter using Neutron Activation Analysis and Inductively Coupled Plasma Atomic Emission Spectrometry. International Journal of Medical and All Body Health Research 2(2): 5-18.
- Zaichick V (2021) Comparison of twenty chemical element contents in normal thyroid tissue and hypertrophic thyroid tissue. Universal Journal of Pharmaceutical Research 6(4): 32-42.
- Zaichick V (2021) Evaluation of Twenty Chemical Element Contents in Thyroid Adenomas using Neutron Activation Analysis and Inductively Coupled Plasma Atomic Emission Spectrometry. World Journal of Advanced Research and Reviews 11(03): 242-257.
- Zaichick V (2021) Are there any Differences between Chemical Element Contents of Goitrous and Adenomatous Thyroid? Journal of Medical Research and Health Sciences 4 (11):1576-1587.
- Zaichick V, Zaichick S (1996) Instrumental effect on the contamination of biomedical samples in the course of sampling. The Journal of Analytical Chemistry 51(12):1200-1205.
- Zaichick V, Zaichick S (1997) A search for losses of chemical elements during freeze-drying of biological materials. J Radioanal Nucl Chem 218(2): 249-253.
- Zaichick S, Zaichick V (2011) INAA application in the age dynamics assessment of Br, Ca, Cl, K, Mg, Mn, and Na content in the normal human prostate. J Radioanal Nucl Chem 288: 197-202.
- Zaichick V, Zaichick S (2013) The effect of age on Br, Ca, Cl, K, Mg, Mn, and Na mass fraction in pediatric and young adult prostate glands investigated by neutron activation analysis. J Appl Radiat Isot 82: 145-151.
- Zaichick V, Nosenko S, Moskvina I (2012) The effect of age on 12 chemical element contents in intact prostate of adult men investigated by inductively coupled plasma atomic emission spectrometry. Biol Trace Elem Res 147: 49-58.
- Zaichick V, Zaichick S (2013) NAA-SLR and ICP-AES Application in the assessment of mass fraction of 19 chemical elements in pediatric and young adult prostate glands. Biol Trace Elem Res 156: 357-366.
- Zaichick V, Zaichick S (2014) Determination of trace elements in adults and geriatric prostate combining neutron activation with inductively coupled plasma atomic emission spectrometry. Open Journal of Biochemistry 1(2): 16-33.
- Zaichick S, Zaichick V (2010) The effect of age and gender on 37 chemical element contents in scalp hair of healthy humans. Biol Trace Elem Res 134(1): 41-54.
- Zaichick V (1995) Applications of synthetic reference materials in the medical Radiological Research Centre. Fresenius J Anal Chem 352: 219-223.
- Korelo AM, Zaichick V (1993) Software to optimize the multielement INAA of medical and environmental samples. In: Activation Analysis in Environment Protection. Dubna, Russia: Joint Institute for Nuclear Research 326-332.
- Leko MB, Gunjača I, Pleić N, Zemunik T (2021) Environmental Factors Affecting Thyroid-Stimulating Hormone and Thyroid Hormone Levels. Int J Mol Sci 22(12): 6521.
- Schwartz GG, Klug MG (2019) Thyroid Cancer Incidence Rates in North Dakota are Associated with Land and Water Use. Int J Environ Res Public Health 16(20): pp.3805.
- National Toxicology Program (2005) Toxicology and carcinogenesis studies of sodium chlorate (Cas No. 7775-09-9) in F344/N rats and B6C3F1 mice (drinking water studies). Natl Toxicol Program Tech Rep Ser 517: 1-255.
- Parazzini F, Esposito G, Tozzi L, Tozzi S (2017) Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol 209: 3-7.
- Sokal A, Jarmakiewicz Czaja S, Tabarkiewicz J, Filip R (2021) Dietary Intake of Endocrine Disrupting Substances Presents in Environment and Their Impact on Thyroid Function. Nutrients 13(3): pp. 867.
- Zaichick V (1998) X-ray fluorescence analysis of bromine for the estimation of extracellular water. J Appl Radiat Isot 49(12):1165-1169.
- Manz DH, Blanchette NI, Paul BT, Torti FM, Torti SV (2016) Iron and cancer: recent insights. Ann N Y Acad Sci 1368(1): 149-161.
- Torti SV, Manz DH, Paul BT, Blanchette Farra N, Torti FM (2018) Iron and cancer. Annu Rev Nutr 38: 97-125.
- Selby JV, Friedman GD (1988) Epidemiologic evidence of an association between body iron stores and risk of cancer. Int J Cancer 41: 677-682.
- Meneghini R (1997) Iron homeostasis, oxidative stress, and DNA damage. Free Radic Biol Med 23:783-792.
- Razy NHMP, Rahman WFWA, Win TT (2019) Expression of vascular endothelial growth factor and its receptors in thyroid nodular hyperplasia and papillary thyroid carcinoma: A Tertiary Health Care Centre based study. Asian Pac J Cancer Prev 20(1):277-282.
- Kant R, Davis A, Verma V (2020) Thyroid nodules: Advances in evaluation and management. Am Fam Physician 102(5): 298-304.
- Zaichick V, Zaichick S (2018) Variation in selected chemical element contents associated with malignant tumors of human thyroid gland. Cancer Studies 2(1): 1-12.
- Zaichick V, Zaichick S (2018) Twenty chemical element contents in normal and cancerous thyroid. Int J Hematol Blo Dis 3(2): 1-13.
- Zaichick V, Zaichick S (2018) Levels of chemical element contents in thyroid as potential biomarkers for cancer diagnosis (a preliminary study). J Cancer Metastasis Treat 4:1-15.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

.png)


