
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Case Report(ISSN: 2637-6679) 
Congenital Thrombotic Thrombocytopenic Purpura Due to A Novel Homozygous Mutation in The ADAMTS13 Gene: A Case Report Volume 7 - Issue 3
Housam Yousef Almadani1*, Mohammed Abdulbasit Almelibari2, Mohamed I El hefnawy³, Banan Alaied4, Talal H Albishri5, Samy Marouf6, Ebtihaj Jamil Abdulqadim7, Antoine Amaury Lachaud8, Rita Dittmer9 and Pierre Alain Menoud10
- 1Department of Onco Haematology, King Fahd Armed Forces Hospital, Saudi Arabia
- 2Department of Pediatric, King Fahd Armed Forces Hospital, Saudi Arabia
- 3Department of Onco Haematology, King Fahd Armed Forces Hospital, Saudi Arabia
- 4Department of Ophthalmology, King Fahd Armed Forces Hospital, Saudi Arabia
- 5Department of Laboratory, Laboratory Director, King Fahd Armed Forces Hospital, Saudi Arabia
- 6Department of Onco Haematology, King Fahd Armed Forces Hospital, Saudi Arabia
- 7Department of Laboratory, Unilabs Coordinator, King Fahd Armed forces Hospital, Saudi Arabia
- 8Unilabs Middle East, Dubai UAE, UAE
- 9Asklepios MVZ Nord GmbH, Hamburg, Germany
- 10Unilabs Genetic Laboratory, Lausanne Switzerland
Received: February 15, 2022; Published: February 28, 2022
Corresponding author: Housam Yousef Almadania, Department of Onco Haematology, King Fahd Armed Forces Hospital, Saudi Arabia
DOI: 10.32474/RRHOAJ.2022.07.000265
Abstract
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening form of thrombotic microangiopathy (TMA) characterized by recurrent episodes of thrombocytopenia and microangiopathic hemolytic anemia (MAHA), sometimes associated with ischemic damage of the brain and kidneys. Congenital TTP (cTTP), also known as Upshaw–Schulman syndrome, is a rare disease caused by compound heterozygous, or more rarely, homozygous mutations in the gene encoding the von Willebrand factor (VWF) cleaving protease, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). The disease course in cTTP is variable and genotype-phenotype correlations are complex. In this report we describe a case of cTTP in a patient from Saudi Arabia caused by a novel homozygous mutation (c.2882delC; p. Cys962Alafs*3) predicted to result in truncation of a third of the C-terminal region of the ADAMTS13 protein, including the distal TSP-1 and CUB domains. The cTTP in our patient was characterized by severe ADAMTS13 deficiency (<2%), early onset (severe neonatal hyperbilirubinemia) recurrent episodes of thrombocytopenia and MAHA, requiring prophylactic treatment with fresh frozen plasma infusions. No neurological or renal manifestations were present. This case report provides further evidence that both the distal TSP-1 and CUB domains are essential for the VWF-cleaving activity of ADAMTS13.
Keywords: Congenital thrombotic thrombocytopenic purpura; Upshaw–Schulman syndrome; ADAMTS13; von Willebrand factor
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a lifethreatening form of thrombotic microangiopathy (TMA) characterized by recurrent episodes of thrombocytopenia and microangiopathic hemolytic anemia (MAHA), with widespread microvascular thrombosis leading to ischemic damage of multiple organs, predominantly the brain and kidneys [1-3]. TTP is caused by inherited or acquired deficiency of the von Willebrand factor (VWF)-cleaving protease, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). The congenital form (cTTP), also known as Upshaw–Schulman syndrome, is caused by inherited mutations in the ADAMTS13 gene [4], whereas the acquired form is associated with the presence of circulating inhibitory anti-ADAMTS13 autoantibodies [5,6]. ADAMTS13 deficiency results in persistence of unusually large VWF multimers in the circulation, leading to spontaneous platelet aggregation and the formation of microvascular thrombi and fragmentation of erythrocytes (MAHA, [1]). Congenital TTP is a rare autosomal recessive disease, with an estimated annual incidence of less than 1/million inhabitants [7]. The clinical phenotype ranges from patients with neonatal onset with frequent reoccurrences of thrombocytopenia and MAHA and multiorgan failure, to patients with adult onset and isolated episodes of TTP [7-11]. Episodes may be triggered by precipitating factors such as infections, surgery and pregnancy [8,9,12]. Infusions of fresh frozen plasma (FFP) are the mainstay of treatment, with prophylactic FFP infusions every 2-3 weeks being sufficient to prevent episodes in patients with severe relapsing disease ([3,13-15]. Over 150 ADAMTS13 mutations have been identified so far, distributed throughout the entire gene [11]. However, the majority of cases described in the literature are associated with compound heterozygous mutations, with homozygous mutations identified in only 36% of reported patients [16]. Here, we describe a patient from Saudi Arabia with a novel homozygous mutation in the ADAMTS13 gene resulting in severe ADAMTS13 deficiency and early-onset cTTP, without renal or neurological manifestations.
Case Presentation
Figure 1: Peripheral blood smear revealing numerous schistocytes, anisopoikilocytosis, polychromasia and a few erythroblasts. Giant platelets with normal granulation were also observed.
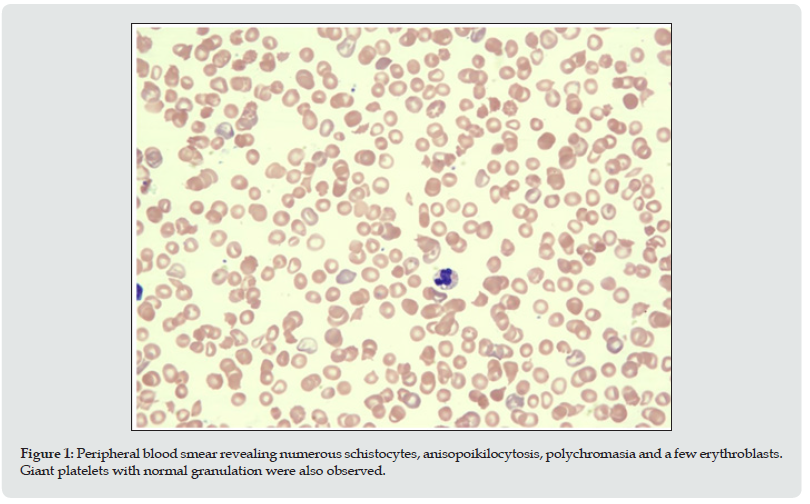
The proband was a 2-year-old girl referred to the hematology department of the King Fahd Armed Forces Hospital, Jeddah, Saudi Arabia in September 2014 for pancytopenia, in the absence of thrombosis, or any neurological or renal manifestations. The patient was born at term (at 40 weeks’ gestation; birth weight: 3.095 kg) to consanguineous parents. Meconium staining was noted at birth, and she was transferred to the neonatal intensive care unit for severe neonatal hyperbilirubinemia, requiring treatment with intensive phototherapy. MRI revealed increased signal intensity in the globus palledus. One month later, she was referred to the hematology department for thrombocytopenia, for which two rounds of intravenous immunoglobulin (IVIG) were administered before the patient was then discharged. The patient also had a history of multiple food allergies. Further hematological investigation of the pancytopenia at 2 years of age revealed anemia (Hb 7.1 g/dL), severe thrombocytopenia (platelet count 99 x 109 L), a white blood cell count of 3.1/μL and reticulocytosis. Lactate dehydrogenase levels were elevated (1358 U/L). The DEB test was negative. Fragmented erythrocytes (schistocytes estimated at 12- 150%) were observed on the peripheral blood smear (Figure 1). These findings are consistent with a diagnosis of microangiopathic hemolytic anemia. Examination of the bone marrow revealed erythroid hyperplasia. No circulating antiplatelet antibodies directed against glycoprotein complexes IIb-IIIa, Ia-IIa and Ib-IX were detected, precluding the existence of autoimmune hemolytic anemia. Normal renal function, absence of a history of diarrhea and negative stool cultures appeared to exclude a diagnosis of hemolytic uremic syndrome (HUS). ADAMTS13 activity was measured by ELISA, using a commercial kit (Technoclone GmbH, Vienna Austria), in an international reference laboratory for ADAMTS13 diagnostics (Asklepios MVZ Nord GmbH, Hamburg, Germany). The results revealed a severe ADAMTS13 deficiency, with <2 % residual activity, and were strongly indicative of TTP. The diagnosis of cTTP was confirmed by genetic analysis of the ADAMTS13 gene, which revealed the presence of a homozygous mutation involving the single nucleotide deletion c.2882delC. This mutation was not listed in either the Exome Aggregation Consortium (ExAC) or dbsNP databases. This novel mutation led to a frameshift (p. Cys962Alafs*3) and introduction of a premature stop codon that resulted in truncation of a third of the C-terminal portion of the protein. The same mutation was identified in a heterozygous state in the both the mother and father. Following diagnosis of cTTP the patient is being treated with regular infusions of solvent/detergent treated FFP (10 ml/kg, every 2-3 weeks).
Discussion
We have identified a novel homozygous ADAMTS13 mutation (c.2882delC; p. Cys962Alafs*3) in a patient from Saudi Arabia that led to truncation of a third of the C-terminal part of the protein and resulted in early-onset cTTP associated with severe ADAMTS13 deficiency (level <2%). To our knowledge, this is only the third cTTP-associated ADAMTS13 mutation to be reported in a family from Saudi Arabia. The 37kb ADAMTS13 gene is located on chromosome 9q34 and contains 29 exons, encoding a 180 kDa (1427 amino acid) protein, with an N-terminal signal peptide, a propeptide, a metalloprotease domain, a disintegrin-like domain, a thrombospondin-type 1 (TSP-1) motif, a cysteine-rich domain and a spacer domain, followed by a further seven TSP-1 repeats and two CUB (complement components C1r and C1s, sea urchin protein UEGF and bone morphogenetic protein) domains at the C-terminus [4,17]. The homozygous 2882delC mutation identified in our patient resulted in substitution of Cys962 for Ala in the sixth TSP-1 (TSP-1-6) domain in the C-terminal portion of ADAMTS13, causing a frameshift and introduction of stop codon that is predicted to lead to production of a truncated protein lacking the remaining TSP-1 repeats and the CUB domains. Genotype-phenotype studies have suggested that mutations in the N-terminal portion of ADAMTS13 are more likely to be associated with severe cTTP (earlier onset, organ damage, requirement for prophylactic FFP) than those involving the C-terminal domains [8,18]. The C-terminal TSP-1-7, TSP-1-8 and CUB domains have been found to be dispensable for ADAMTS13-mediated cleavage of VWF under static conditions [19], however, several studies have indicated that these distal TSP- 1 domains play an important role in modulating the interaction between ADAMTS13 and VWF [20-23]. Indeed, more recent studies have shown that the C-terminal ADAMTS13 fragments display antithrombotic activity, and that this activity is mediated, independently of proteolytic activity, by disulfide-bond-reducing activity localized to the C-terminal TSP1 2–8 and CUB domains of ADAMTS13 [24,25]. Our case report, together with other previously reported mutations in the distal TSP-1 and CUB domains that result in clinically overt cTTP [26-30], highlight the important physiological role played by these domains in ADAMTS13-mediated cleavage of VWF.
The C-terminal truncation identified in our patient resulted in an early-onset form of cTTP. Although the patient described in this report was diagnosed with cTTP at the age of 2 years, onset of the disease is likely to have occurred at birth with severe neonatal hyperbilirubinemia. Severe hyperbilirubinemia is one of the classic hallmarks of cTTP, occurring in 42-45% of patients, and often requiring exchange transfusion [9,31]. In our patient, intensive phototherapy was sufficient for resolution of the hyperbilirubinemia. The neonatal onset observed in this case was associated with a severe ADAMTS13 deficiency (level <2%). Although genotype-phenotype correlations in cTTP remain complex, there appears to be a correlation between residual plasma ADAMTS13 activity and the severity of the disease course [8]. This correlation was clearly observed in our patient where the severe ADAMTS13 deficiency resulted in a cTTP phenotype characterized by neonatal onset, occurrence of three TTP episodes by the age of 2 years and dependency on FFP prophylaxis. Other studies have suggested that mutations leading to early onset cTTP and low residual ADAMTS13 activity are more likely to be associated with renal and neurological impairment [32,33], however, renal and CNS manifestations were not observed in our patient. Due to the potentially fatal nature of this disease, clinical guidelines now recommend that a preliminary diagnosis of cTTP and rapid initiation of FFP infusions should be considered in all patients with MAHA and thrombocytopenia of unknown cause, even in the absence of renal or neurological manifestations [34].
ADAMTS13 mutations associated with cTTP have been reported previously in two families of Saudi origin [35]. A homozygous deletion (c.1922delA, p. E641GfsX57) in the N-terminal cysteinerich domain was identified in a female cTTP patient in one family, whilst homozygous deletions (c.1649_1664del, p. G550Afs) in the spacer domain were observed in two brothers from the second family. Both mutations led to frameshifts and truncation of ADAMTS13. The phenotypes observed in these patients were very similar to that described in our study, with all patients manifesting neonatal hyperbilirubinemia and onset of cTTP episodes during early childhood (1-4 years of age). Prophylactic FFP was required in all patients, however, as in our case, renal and neurological complications were not observed. Further studies are required to evaluate the natural history and spectrum of ADAMTS13 mutations in cTTP patients in Saudi Arabia. In summary, this case report provides the first description of a cTTP phenotype caused by a novel homozygous ADAMTS13 mutation (c.2882delC; p. Cys962Alafs*3) resulting in truncation of the C-terminus of the ADAMTS13 protein, with loss of the distal TSP-1 and CUB domains. This mutation led to neonatal onset of cTTP with a severe reduction of ADAMTS13 activity to below 2% of normal levels but was not associated with renal or neurological impairment. This is only the fourth case of cTTP from Saudi Arabia described in the literature. The early onset cTTP phenotype and severe ADAMTS13 deficiency described in our patient highlights the essential role played by the C-terminal ADAMTS13 domains in the blinding and cleavage of VWF but further studies are required to determine the mechanism by which the mutation described in our patient results in ADAMTS13 deficiency.
References
- Moake JL (2002) Thrombotic microangiopathies. N Engl J Med 347 (8): 589-600.
- Zheng XL, Sadler JE (2008) Pathogenesis of Thrombotic Microangiopathies. Annual review of pathology 3: 249-277.
- Loirat C, Girma JP, Desconclois C, Coppo P, Veyradier A (2008) Thrombotic thrombocytopenic purpura related to severe ADAMTS13 deficiency in children. Pediatric Nephrology 24 (1): 19-29.
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, et al. (2001) Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 413 (6855): 488-494.
- Tsai HM, Lian EC (1998) Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med 339 (22): 1585-1594.
- Furlan M, Robles R, Solenthaler M, Lammle B (1998) Acquired deficiency of von Willebrand factor-cleaving protease in a patient with thrombotic thrombocytopenic purpura. Blood 91(8): 2839-2846.
- Pérez Rodríguez A, Lourés E, Rodríguez Trillo Á, Costa Pinto J, García Rivero A, Batlle López A, et al. (2014) Inherited ADAMTS13 deficiency (Upshaw-Schulman syndrome): A short review. Thrombosis Research 134 (6):1171-1175.
- Lotta LA, Wu HM, Mackie IJ, Noris M, Veyradier A, Scully MA, Remuzzi G, Coppo P, Liesner R, Donadelli R, Loirat C, Gibbs RA, Horne A, Yang S, Garagiola I, Musallam KM, Peyvandi F (2012) Residual plasmatic activity of ADAMTS13 is correlated with phenotype severity in congenital thrombotic thrombocytopenic purpura. Blood 120(2): 440-448.
- Fujimura Y, Matsumoto M, Isonishi A, Yagi H, Kokame K, Soejima K, Murata M, Miyata T (2011) Natural history of Upshaw–Schulman syndrome based on ADAMTS13 gene analysis in Japan. Journal of Thrombosis and Haemostasis 9: 283-301.
- Camilleri RS, Scully M, Thomas M, Mackie IJ, Liesner R, Chen WJ, Manns K, Machin SJ (2012) A phenotype-genotype correlation of ADAMTS13 mutations in congenital thrombotic thrombocytopenic purpura patients treated in the United Kingdom. Journal of thrombosis and haemostasis: JTH 10 (9): 1792-1801.
- Mansouri Taleghani M, von Krogh AS, Fujimura Y, George JN, Hrachovinova I, Knobl PN, Quist-Paulsen P, Schneppenheim R, Lammle B, Kremer Hovinga JA (2013) Hereditary thrombotic thrombocytopenic purpura and the hereditary TTP registry. Hamostaseologie 33 (2): 138-143.
- Furlan M, Lammle B (2001) Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome: the role of von Willebrand factor-cleaving protease. Best practice & research Clinical haematology 14(2): 437-454.
- Furlan M, Robles R, Morselli B, Sandoz P, Lammle B (1999) Recovery and half-life of von Willebrand factor-cleaving protease after plasma therapy in patients with thrombotic thrombocytopenic purpura. Thrombosis and haemostasis 81(1): 8-13
- Barbot J, Costa E, Guerra M, Barreirinho MS, Isvarlal P, et al. (2001) Ten years of prophylactic treatment with fresh-frozen plasma in a child with chronic relapsing thrombotic thrombocytopenic purpura as a result of a congenital deficiency of von Willebrand factor-cleaving protease. Br J Haematol 113(3): 649-651.
- Hanby HA, Zheng XL (2014) Current status in diagnosis and treatment of hereditary thrombotic thrombocytopenic purpura. Hereditary genetics: current research 3(1): e108.
- Lotta LA, Garagiola I, Palla R, Cairo A, Peyvandi F (2010) ADAMTS13 mutations and polymorphisms in congenital thrombotic thrombocytopenic purpura. Human mutation 31(1): 11-19.
- Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K (2001) Structure of von Willebrand Factor-cleaving Protease (ADAMTS13), a Metalloprotease Involved in Thrombotic Thrombocytopenic Purpura. Journal of Biological Chemistry 276(44): 41059-41063.
- Hing ZA, Schiller T, Wu A, Hamasaki Katagiri N, Struble EB, et al. (2013) Multiple in silico tools predict phenotypic manifestations in congenital thrombotic thrombocytopenic purpura. Br J Haematol 160(6): 825-837.
- Zheng X, Nishio K, Majerus EM, Sadler JE (2003) Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. The Journal of biological chemistry 278(32): 30136-30141.
- Tao Z, Peng Y, Nolasco L, Cal S, Lopez-Otin C, Li R, Moake JL, Lopez JA, Dong JF (2005) Recombinant CUB-1 domain polypeptide inhibits the cleavage of ULVWF strings by ADAMTS13 under flow conditions. Blood 106(13): 4139-4145.
- Zhang P, Pan W, Rux AH, Sachais BS, Zheng XL (2007) The cooperative activity between the carboxyl terminal TSP1 repeats and the CUB domains of ADAMTS13 is crucial for recognition of von Willebrand factor under flow. Blood 110(6): 1887-1894.
- Majerus EM, Anderson PJ, Sadler JE (2005) Binding of ADAMTS13 to von Willebrand Factor. Journal of Biological Chemistry 280(23): 21773-21778.
- Dong Jf, Moake JL, Bernardo A, Fujikawa K, Ball C, Nolasco L, López JA, Cruz MA (2003) ADAMTS-13 Metalloprotease Interacts with the Endothelial Cell-derived Ultra-large von Willebrand Factor. Journal of Biological Chemistry 278(32): 29633-29639.
- Yeh HC, Zhou Z, Choi H, Tekeoglu S, May W, 3rd Wang C, Turner N, Scheiflinger F, Moake JL, Dong JF (2010) Disulfide bond reduction of von Willebrand factor by ADAMTS-13. Journal of thrombosis and haemostasis JTH 8(12): 2778-2788.
- Bao J, Xiao J, Mao Y, Zheng XL (2014) Carboxyl terminus of ADAMTS13 directly inhibits platelet aggregation and ultra large von Willebrand factor string formation under flow in a free-thiol-dependent manner. Arteriosclerosis, thrombosis, and vascular biology 34(2): 397-407.
- Banno F, Kaminaka K, Soejima K, Kokame K, Miyata T (2004) Identification of Strain-specific Variants of Mouse Adamts13 Gene Encoding von Willebrand Factor-cleaving Protease. Journal of Biological Chemistry 279(29): 30896-30903.
- Kim HY, Lee KO, Yoo KH, Kim SH, Oh D, Kim HJ (2016) Congenital thrombotic thrombocytopenic purpura (Upshaw-Schulman syndrome) caused by novel ADAMTS13 mutations. Br J Haematol 173(1): 156-159.
- Rossio R, Ferrari B, Cairo A, Mancini I, Pisapia G, Palazzo G, Peyvandi F (2013) Two novel heterozygote missense mutations of the ADAMTS13 gene in a child with recurrent thrombotic thrombocytopenic purpura. Blood Transfusion 11(2): 241-244.
- Tanabe S, Yagi H, Kimura T, Isonishi A, Kato S, Yoshida Y, Hayakawa M, Matsumoto M, Ohtaki S, Takahashi Y, Fujimura Y (2012) Two newborn-onset patients of Upshaw-Schulman syndrome with distinct subsequent clinical courses. International journal of hematology 96(6): 789-797.
- Palla R, Lavoretano S, Lombardi R, Garagiola I, Karimi M, Afrasiabi A, Ramzi M, De Cristofaro R, Peyvandi F (2009) The first deletion mutation in the TSP1-6 repeat domain of ADAMTS13 in a family with inherited thrombotic thrombocytopenic purpura. Haematologica 94(2): 289-293.
- Sarode R, Bandarenko N, Brecher ME, Kiss JE, Marques MB, Szczepiorkowski ZM, Winters JL (2014) Thrombotic thrombocytopenic purpura: 2012 American Society for Apheresis (ASFA) consensus conference on classification, diagnosis, management, and future research. Journal of Clinical Apheresis 29(3): 148-167.
- Rurali E, Banterla F, Donadelli R, Bresin E, Galbusera M, Gastoldi S, Peyvandi F, Underwood M, Remuzzi G, Noris M (2015) ADAMTS13 Secretion and Residual Activity among Patients with Congenital Thrombotic Thrombocytopenic Purpura with and without Renal Impairment. Clinical journal of the American Society of Nephrology: CJASN 10(11): 2002-2012.
- Berti de Marinis G, Novello S, Ferrari S, Barzon I, Cortella I, et al. (2016) Correlation between ADAMTS13 activity and neurological impairment in acute thrombotic microangiopathy patients. Journal of thrombosis and thrombolysis 42(4): 586-592.
- Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F, Cheung B, Machin SJ, on behalf of British Committee for Standards in H (2012) Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. British Journal of Haematology 158(3): 323-335.
- Alsultan A, Jarrar M, Al-Harbi T, M ALB (2013) Novel frameshift mutations in ADAMTS13 in two families with hereditary thrombotic thrombocytopenic purpura. Pediatr Blood Cancer 60(9): 1559-1560.

.png)


