
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Research Article(ISSN: 2637-6679) 
A Rare Case of Hemophagocytic Lymphohistiocytosis in a Young Adult Male with Mediastinal Mixed Germ Cell Tumor Volume 4 - Issue 5
Onyedika Umeanaeto MD1*, M Nawar Hakim MD2, Jesus Diaz MD3, Charlene Ofosu MD3 and Sumit Gaur MD1
- 1Department of Internal Medicine, Paul L Foster School of Medicine, USA
- 2Department of Pathology, , Paul L Foster School of Medicine, USA
- 3Department of Radiology, Paul L Foster School of Medicine, USA
Received: February 24, 2020; Published: March 04, 2020
Corresponding author: Onyedika Umeanaeto MD, Resident Physician, Department of Internal Medicine TTUHSC-El Paso. 4800 Alberta Avenue, El Paso, TX 79905. USA
DOI: 10.32474/RRHOAJ.2020.04.000200
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life threatening and fatal medical condition which usually results in death in patients that are affected even with adequate and proper medical care. Due to how uncommon this condition is, delay in diagnosis is most often the major determinant of prognosis since in many cases patient are diagnosed late in their hospital stay. It most frequently affects infants from birth to 18 months of age, but the disease is also observed in children and adults of all ages. HLH can occur as a familial or sporadic disorder, and it can be triggered by a variety of events that disrupt immune homeostasis. Infection is a common trigger both in those with a genetic predisposition and in sporadic cases. We present a case of a 20-year-old Hispanic male who presented with a mediastinal germ cell tumor and was found to have HLH. He underwent HLH genetic testing which were all negative, making us to rule out primary HLH in this case. Infections and malignancies are the most common triggers of HLH both in familiar and sporadic cases. Malignancy associated HLH are not uncommon medical conditions, however there is a unique association between hematological mediastinal tumors and HLH; and reported cases have shown that prognosis is usually worse when HLH is associated with a malignancy.
Case Presentation
Patient is 20year old male with no previous past medical history presenting due to cough, fevers and anterior chest pain. Two months ago, patient started having productive whitish cough, intermittent right sided chest and generalized arthralgias. He also admitted about 10 pounds weight loss since symptoms onset. Past medical and surgical histories are unremarkable. He denied alcohol, smoking, illicit and/or intra-venous (IV) drug use. Family history is unremarkable, and he takes no medication and has no allergies on exam, patient is pale looking, temperature was 39.4 with a resting pulse rate of 130, blood pressure 164/80, and oxygen saturation 96% on room air. Chest has symmetrical expansion, non-tender to palpation, with no obvious chest wall deformity. Breath sounds were clear bilaterally, with no murmurs or extra heart sounds on cardiac auscultation. No edema or tenderness to scrotum, no palpable mass or tenderness to testicles, with normal appearance and development of the penis. He appeared mildly anxious, but otherwise rest of physical exam is unremarkable. Laboratory on admission showed white blood cell (WBC) 3.24 cells/ul (4.50-11.00), hemoglobin 8.7gm/dl, (12.0-16.0), hematocrit 28.2% (38.0-47.0), normal mean corpuscular volume (MCV), platelet 43 cells/ul (150-450), iron 14mcg/dl (49-181), ferritin 3380ng/ml (17.90-464.00). Serology for human immunodeficiency virus (HIV) and hepatitis viruses were all negative. Liver enzymes were normal on admission but worsened few days after admission to a peak of alkaline phosphatase (ALK) 474 unit/L (38-126), aspartate aminotransferase (AST) 327 unit/L (17-59) and alanine aminotransferase (ALT) 460 unit/L (21-72) Chest X-ray on admission showed large right midlung zone opacity with silhouetting of the right heart border suggestive of anterior mediastinal mass. Scrotal ultrasound (US) showed severe degree bilateral testicular microlithiasis. Computed Tomography (CT) chest with IV contrast showed large heterogeneous anterior mediastinal mass measuring 10 x 10 x 12 cm (Figure 1). CT abdomen with intravenous contrast only showed hepatosplenomegaly. Remainder of laboratory workup showed serum lactate dehydrogenase (LDH) 255unit/L (120-246), triglycerides 301 mg/dl (35-150), alpha fetoprotein (AFP) 2070ng/ml (0.00-7.51), and serum quantitative human chorionic gonadotropin (HCG) 187mIU/ml (0-4.32 for males). Patient underwent CT-guided core needle biopsy of the mediastinal mass by interventional radiology (IR) with pathology result consistent with stage IIIb mixed germ cell tumor (Figure 2-4).
Figure 1: CT Thorax/ Chest with IV contrast axial and coronal images (A and B) demonstrates a 10 x 10 x 12 cm (AP x TR x CC) heterogeneous anterior mediastinal mass with an irregularly enhancing peripheral rim without internal calcification extending from the thoracic inlet to the right hemidiaphragm and right cardiophrenic angle. There is posterior displacement of the superior vena cava (arrow). CT Thorax/ Chest with IV contrast axial and coronal images (C and D) performed after one cycle of chemotherapy five weeks after initial CT shows no significant change in heterogeneous anterior mediastinal mass with an irregularly enhancing peripheral rim without internal calcification extending from the thoracic inlet to the right hemidiaphragm and right cardiophrenic angle.
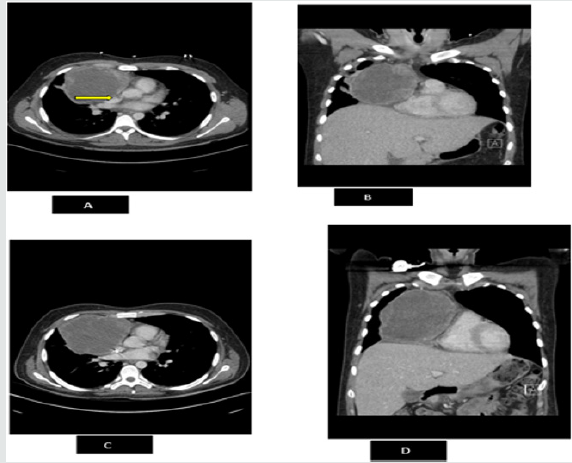
Figure 2: Section of the mediastinal tumor shows a mixture of seminoma (long arrow) and yolk sac tumor (two short arrows). H&E stain, original magnification X100.
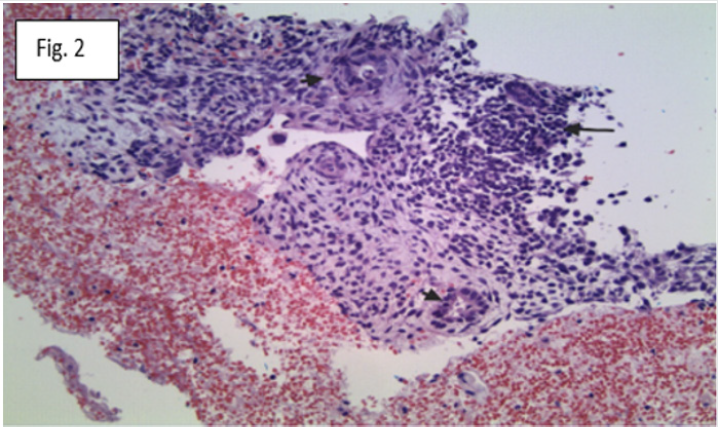
Figure 3:CD117 immunohistochemical stain accentuates the seminoma component (arrow). Original magnification X200.
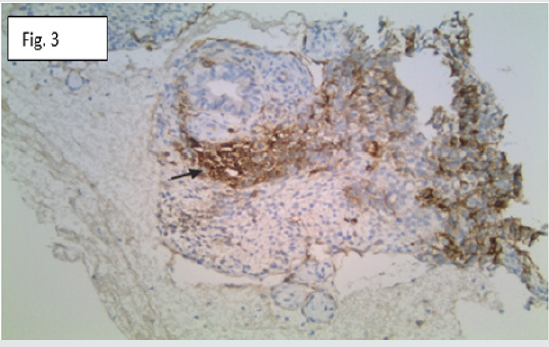
Figure 4: AFP immunohistochemical stain accentuates the yolk sac component (arrows). Original magnification X100.
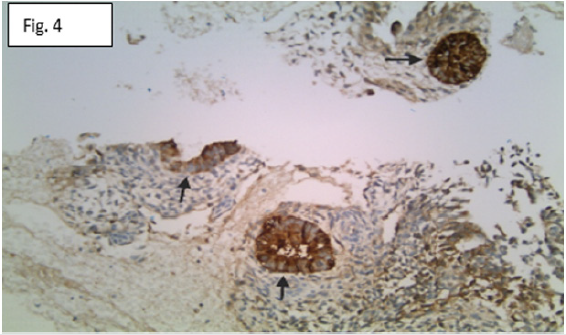
Figure 5: The bone needle core biopsy shows a normocellular marrow for the patient’s age (H&E stain, original magnification X100).
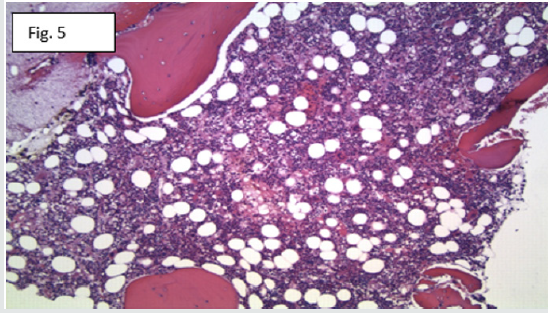
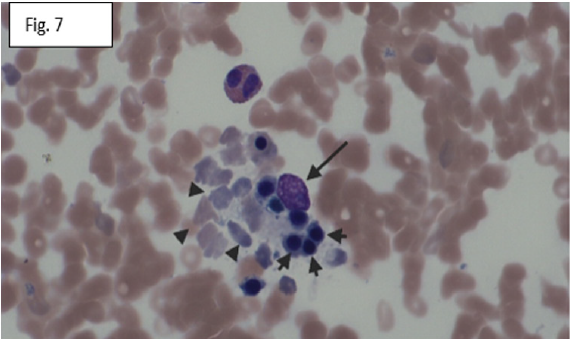
He was emergently started on infusional dose adjusted BEP (bleomycin, etopoxide, and cisplatinum). Concern for hemophagocytic lymphohistiocytosis (HLH) was suspected at admission and to rule out other causes of HLH, further studies were done which all came out negative, as follows: Epstein - Barr virus (EBV), cytomegalovirus (CMV), antinuclear antibody (ANA), rheumatoid factor (RF), anti-cyclic citrullinated peptide (anti-CCP), direct platelet antibody, antineutrophil cytoplasmic antibodies (ANCA), cardiolopin antibodies (Immunoglobulin G, Immunoglobulin A, and Immunoglobulin M), coccidiodes antibody, heparin induced platelet antibody, and aspergillus antigen. Patient’s interleukin-2 receptor (IL-2) was severely elevated at 28676 pg/ mL (532 - 1891). He underwent bone marrow biopsy, which was normocellular for the patient’s age, but showed several phagocytes, some of which contain phagocytized red blood cells and other hematopoietic elements (Figure 5-7). Patient’s H-score for HLH was calculated at 254 (cutoff of 169 and above), with a more than 99% probability of having HLH. He was started on high dose intravenous steroids (dexamethasone 10mg every 24hours) with continuation of BEP chemotherapy. Patient completed first cycle of BEP, his AFP improved to 306 ng/ml (from 2070 on admission), HCG 54mIU/ ml (from 187 on admission), however repeat CT chest showed no change in the size of the mediastinal mass Figure 1. He completed first course of BEP chemotherapy and cardiothoracic surgery was consulted for possible resection of the mediastinal mass. His pancytopenia persisted and worsened upon commencement of chemotherapy with nadir WBC 0.06 cells/ul, hemoglobin 5.8gm/ dl, and platelet 2 cells/ul. Patient was switched to a different chemotherapy regimen VeIP (vinblastine, ifosfomide and cisplatin) after completion of BEP. He was transfusion dependent with almost daily transfusions of platelet, fresh frozen plasma, and packed red blood cells for two months of his hospital stay at our facility. Repeat CT chest with IV contrast after completion of second cycle of chemotherapy continued to show no change in the size of the mediastinal mass despite continued decrease in patient’s tumor markers- AFP and HCG levels (normalized at this point). Patient had to be transferred to another facility for a higher level of care, unfortunately he died after three days in that facility despite advanced care.
Figure 6: The bone marrow aspirate smear shows macrophages (long arrow) with phagocytized red blood cell (short arrow). Wright Giemsa stain, original magnification X400.
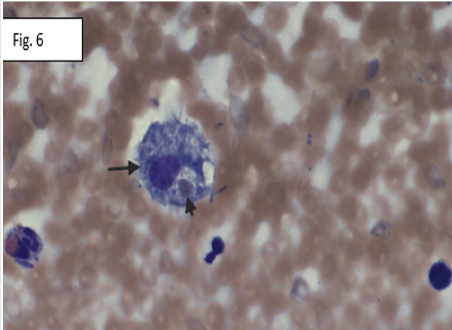
Figure 7: Other macrophage (long arrow) with phagocytized normoblasts (short arrows) and mature red blood cells (arrow heads). Wright Giemsa stain, original magnification X400.
Discussion
Germ cell tumors (GCTs) are classified as extragonadal if there is no evidence of a primary tumor in the testes or ovaries and based upon histology, they are further sub-classified as seminomas (termed dysgerminomas in women), nonseminomatous GCTs (termed nondysgerminomas in women), mature teratomas, and immature teratomas [1]. Extragonadal GCTs typically arise in midline locations, and specific sites vary with age. In adults, the most common sites, in order of frequency, are the anterior mediastinum, the retroperitoneum, and the pineal and suprasellar regions. In infants and young children, the sacrococcygeal and intracranial GCTs are most common. Hemophagocytic lymphohistiocytosis (HLH) is an aggressive and life-threatening syndrome of excessive immune activation. “Macrophage activation syndrome” (MAS), is the principal pathogenesis of HLH and this is the most serious and life-threatening complication of systemic onset juvenile idiopathic arthritis. MAS was first described by Hadchouel et al. as a hemorrhagic syndrome associated with mental status changes, hepatosplenomegaly, increased serum concentrations of liver enzymes, and a sharp fall in blood counts. The term MAS was coined by Stéphan et al. in reference to the bone marrow histologic findings of numerous, well-differentiated macrophages (or histiocytes) actively phagocytosing hematopoietic elements. Such cells may infiltrate many organs in MAS. Often, the greatest barrier to treatment and a successful outcome for individuals with HLH is a delay in diagnosis. Several aspects of the clinical presentation of HLH contribute to this delay, including the rarity of the syndrome, the variable clinical presentation, and the lack of specificity of the clinical and laboratory findings. HLH is classified into two subgroups: Primary HLH (also known as FHL) is a hereditary immune disorder, whereas secondary HLH develops as a complication in various settings such as infection, malignancy, autoimmune disease, post allogeneic hematopoietic stem cell transplantation (allo HSCT), and drug hypersensitivity. Both primary and secondary HLH can be triggered by infections (most common) or other immune activating events, and gene mutations can be found in individuals of any age and with any family history. In practice, a distinction between primary and secondary HLH is not essential for the initial diagnosis and management, however, identification of a gene mutation may be useful for subsequent management. Five familial HLH disease subtypes (FHL1, FHL2, FHL3, FHL4, and FHL5) are described and four genes in which pathogenic variants are causative have been identified: PRF1 (FHL2), UNC13D (FHL3), STX11 (FHL4), and STXBP2 (FHL5) [2].
The median survival of children with typical FHL, without treatment, is less than two months; with progression of the HLH and infection accounting for the majority of deaths in untreated individuals. The estimated prevalence of FHL is 1:50,000 births Henter et al. with equal gender distribution. A common PRF1 pathogenic variant, p.Leu17ArgfsTer34, has been observed at a high frequency in individuals with FHL in the African American population Lee et al. Malignancy-related HLH has been reported in many literatures and the most common malignancy types are hematological neoplasmas (93.7%) with NK- or T cell malignancies (35.2%), followed by B-cell lymphoma (31.8%), Hodgkin lymphoma (5.8%), acute leukemia (6.4%), and other hematologic neoplasms (14.4%) [3]. When HLH is associated with a malignancy, it is often more immediately life-threatening than the malignancy itself, and patients who eventually die, die from HLH rather than the existing malignancy. Very few cases of HLH associated with germ cell tumor have been reported in literature, and in some of such cases, patient’s HLH developed after commencement of chemotherapy while in other cases, the patients were diagnosed with HLH upon or later during hospital admission. In our literature search, we were not able to find any reported cases were patients with germ cell tumor and HLH had resolution of their HLH as the GCT responded to chemotherapy treatment. So many experts have argued if cisplatin-based chemotherapy is a contributing factor to hematological disorders in patients with GCT. A literature review of the New England Journal of Medicine showed that at a facility in Indiana, hematologic disorders associated with germ cell tumors showed that out of 28 patients with germ cell tumors, HLH accounted for about 25% of all hematological disorders; with almost all patients (both those with HLH and other hematological disorders) eventually dying despite aggressive treatment [4].
Also, just in our patient, despite adequate treatment for his primary cancer and with his tumor makers decreasing, there was no significant change in his HLH status. This makes one to wonder sometimes if these hematological disorders are related to the germ cell tumor, or if they are both independent entities. In our patient also, one can argue that his hematological disorder was not induced by cisplatin based chemotherapy since he initially presented with some signs concerning for HLH- persistent fevers, pancytopenia, elevated LDH and ferritin, and low fibrinogen, with a very high H-score for HLH. However upon commencement and completion of first dose chemotherapy, his tumor markers significantly improved with no significant change to the size of the mass; also his hemodynamics and his pancytopenia continued to worsen which makes one to question if hematological disorders associated with germ cell tumors are worsened by cisplatin based chemotherapy. Also, no other causes such infection, autoimmune disease, FHL, etc was associated with our patient’s HLH. Some experts believe that hematological disorders that are associated with germ cell tumor most likely arise from same cell line. A review of more than 3500 specimens of leukemic tissue, 600 specimens of lymphomatous tissue, and more than 200 specimens of solid tumors at Memorial Sloan-Kettering Hospital (New York) yielded two cases of leukemia in which an i(12p) was found [4]. Both of these patients with leukemia had mediastinal germ-cell tumors. The clear demonstration of the most common karyotypic abnormality of germ-cell tumors in a mediastinal germ-cell tumor and in the associated leukemic blasts suggests that the hematologic neoplasm and the mediastinal germ-cell tumor arose from a common progenitor cell. Although the pathogenetic mechanisms of the development of this syndrome remain unclear, there is little doubt that there is an association of mediastinal germ-cell tumors with malignant hematopoietic disorders [4]. Diagnosis of HLH is often the biggest dilemma upon initial presentation of patients. An HLH scoring system termed H-score was developed by a French physician (Dr. Laurence Fardet) and this scoring system has a very high positive predictive value for HLH [5]. The widely used modern diagnosis of HLH was proposed by the Histiocyte Society in 1987, and the criteria are detailed below:
Diagnosis of HLH can be established if either A or B is fulfilled
A. Molecular diagnosis consistent with HLH (i.e.
identification of any genes associated with familial HLH)
B. Diagnostic criteria for HLH fulfilled (five of the eight
criteria below)
a) Persistent fever
b) Splenomegaly
c) Cytopenias (affecting ≥two of three lineages in the
peripheral blood): Hemoglobin <9 g/dL (in infants <4 weeks:
hemoglobin <10 g/dL), Platelets <100 000/Μl,
Neutrophils <1000/μL
d) Hypertriglyceridemia and/or hypofibrinogenemia:
Fasting triglycerides ≥265 mg/dL, Fibrinogen ≤150 mg/dL
e) Hemophagocytosis in bone marrow or spleen or lymph
nodes
f) Low or absent NK cell activity
g) Ferritin ≥500 ng/mL
h) Soluble IL‐2 receptor ≥2400 U/mL
Our patient met almost all the criteria in the B section.
Given the poor prognosis of individuals with HLH without prompt and effective treatment, it is recommended that treatment be initiated when clinical suspicion is high, even if all recommended studies are incomplete. In general, treatment involves the following:
a) Chemotherapy and immunotherapy to achieve a clinically
stable resolution prior to hematopoietic cell transplantation
(HCT)
b) Antibiotics or antiviral agents to treat or prevent infections
that may have triggered the exaggerated inflammatory response
c) Allogeneic HCT, the only curative therapy, which should
be undertaken in children with confirmed FHL as early in life as
feasible:
d) Presymptomatically if status is confirmed by family
history of clinical HLH, or
e) After achievement of clinical remission in simplex cases
(i.e., a single occurrence in a family)
Although individual findings in HLH may mimic features of
sepsis and multiorgan dysfunction, close follow-up is needed to define the diagnostic criteria for HLH. The degree of abnormality of
inflammatory markers may help to distinguish these disorders. For
example, in one study, the median ferritin was significantly higher in
patients with HLH than in other inflammatory conditions, including
shock and sepsis . Progressively increasing transaminases, bilirubin,
coagulopathy, ferritin, and sCD25 levels as well as deteriorating
respiratory status are poor prognostic signs. As initially discussed,
macrophage activation system (MAS) is the primary mechanism
of HLH. This causes excessive release of cytokines which causes
an extensive immune activating system and resultant multi-organ
damages such as brain, liver, bone marrow, causing coagulopathy,
pancytopenia, persistent fevers etc. The target therapy for HLH
therefore should be an agent that blocks this extensive cascade
of MAS. In 2018, the FDA approved Emapalumab-Izsg (gamifant),
which is an interferon gamma blocker. It blocks both soluble and
receptor-bound interferon gamma. Clinical trials have shown that
gamifant had an overall response rate of 63% (either complete or
partial response) with a median time to response of about 8days
[6,7]. Initially, three-year-survival in children who received HLH-94
therapy was approximately 64% Horne et al. Unfortunately, about
70% of those patients that responded to therapy, still end up having
HSCT [8]. Use of HSCT has improved survival significantly Henter
et al. More recently, reduced intensity regimens prior to HCT have
diminished the early transplant mortality and increased threeyear-
survival rates to 92% Marsh et al.
References
- Wick MR, Perlman EJ, Orazi A, Muller Hermelink HK. Germ cell tumours of the mediastinum. In tumours of the Lung, Pleura, Thymus and Heart, Travis WD, Brambilla E, Muller Hermelink HK, Harris CC (Eds).
- Ramos Casals M, Brito Zeron P, Lopez Guillermo A(2014) Adult haemophagocytic syndrome. Lancet 383: 1503–1516.
- Craig R Nichols, Bruce J Roth , Nyla Heerema , John Griep , and Guido Tricot (1990) Hematologic Neoplasia Associated with Primary Mediastinal Germ-Cell Tumors. N Engl J Med 322(20):1425-1429.
- Kejian Zhang, Alexandra H Filipovich, Judith Johnson, Rebecca A Marshand Joyce Villanueva, MT, MBA. Hemophagocytic Lymphohistiocytosis, Familial
- Gamifant [Prescribing Information]. Stockholm, Sweden: Swedish Orphan Biovitrum AB (2018) Data on file. Stockholm, Swedish Orphan Biovitrum AB, Sweden.
- Michael B Jordan, Carl E Allen, Sheila Weitzman, Alexandra H Filipovich, Kenneth L McClain (2011) How I treat hemophagocytic lymphohistiocytosis. Blood 118(15): 4041-4052.
- Gupta A, Weitzman S, Abdelhaleem M (2008) The role of hemophagocytosis in bone marrow aspirates in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 50(2):192-194.
- Buyse S, Teixeira L, Galicier L (2010) Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med 36(10):1695-1702.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

.png)


