
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Research Article(ISSN: 2637-6679) 
A Phase II Study of The Pineal Hormones Melatonin And 5-Methoxytryptamine Plus Cannabidiol in Association with Angiotensin 1-7 in the Treatment of Advanced Solid Tumour Patients Eligible for The Only Best Supportive Care Volume 6 - Issue 2
Paolo Lissoni, Giorgio Porro, Giusy Messina, Carla Galli, Giuseppe Di Fede, Agnese Valentini1, Ana Cristina Simoese- Silva2 and Daniel Pedro Cardinali3
- Institute of Biological Institute, Milan, Lombardia, Italy
- 1Madonna del Soccorso Hospital, San Benedetto del Tronto, Ascoli Piceno, Italy
- 2Laboratório Interdisciplinar de Investigação Medica, Faculdade de Medicina, UFMG Belo Horizonte, Brazil
- 3Pontificia Universitad Catolica Argentina, Buenos Aires, Argentina
Received: April 08, 2021 Published: April 20, 2021
Corresponding author: Paolo Lissoni, paolo.lissoni@gmx.com, Italy
DOI: 10.32474/RRHOAJ.2021.06.000234
Abstract
The recent advances in the investigation of tumour biology have demonstrated that the human body may produce several molecules provided by a natural anticancer activity without any toxicity, in particular the pineal hormones melatonin (MLT), 5-methoxytryptamine (5-MTT) and pinealine, the endogenous cannabinoids, oxytocin, and angiotensin 1-7 (Ang 1-7). Unfortunately, despite their well confirmed anticancer and non-toxic properties, very few clinical studies have been performed in an attempt to evaluate the potential therapeutic efficacy of the endogenous human anticancer molecules in the treatment of cancer patients, at least of those eligible for the only palliative therapy. Moreover, most studies have been generally limited to the use of the only pineal MLT. The present preliminary study was carried out to evaluate the anticancer efficacy of an oral administration of MLT (100 mg in the dark period) in association with 5-MTT (10 mg in the light period), the cannabinoid agent cannabidiol (CBD) ( 10 mg twice/day) and Ang 1-7 (0.5 mg/twice day) in a group of 14 untreatable advanced or metastatic cancer patients. The clinical response consisted of partial response (PR) in 2/14 (14%), and stable disease (SD) in 8/14 (57%). Then, a disease control (PR + SD) was achieved in 10/14 (71%), whereas the remaining 4/14 (29%) had a progressive disease. Moreover, disease control was associated with a significant increase in lymphocyte-to-monocyte ratio (LMR), by showing that the control of the neoplastic growth is mediated at least in part by an improvement in the antitumor immune status of cancer patients.The treatment was well tolerated in all patients, and in particular no important decline in blood pressure values occurred. On the contrary, a clear improvement in asthenia was obtained in 8/10 (80%) patients with important asthenia prior to study. This preliminary study may suggest that after the failure of the common standard anticancer therapies, the administration of the main endogenous anticancer neuroendocrine molecules, firstly MLT and Ang 1-7, could constitute an alternative approach to cancer patients instead of the simple best supportive care alone.
Keywords: Angiotensin 1-7; Cannabidiol; Melatonin; Metastatic cancer; 5-Methoxytryptamine; Palliative therapy
Introduction
Today it is known that the status of health would substantially depend on the equilibrium between inflammatory and antiinflammatory response, which is the result of the functionless of the cytokine network. Moreover, within the group of more than 40 cytokines, most of them are defined as interleukins (IL), it has appeared that the number of inflammatory cytokines is clearly greater than that of the anti-inflammatory ones, which are essentially represented by IL-10, TGF-beta and IL-35 [1,2], while IL-2 and IL- 12 are characterized by both inflammatory and anti-inflammatory effects [3-5]. The prevalent inflammatory activity of the cytokine network is balanced by two fundamental neuroendocrine systems provided by an important anti-inflammatory action, consisting of the pineal-brain cannabinoid system functional axis [6-10] and the ACE-2 – angiotensin 1-7 (Ang 1-7)-MAS receptor system [11]. In fact, both the pineal hormone melatonin (MLT) [6-8], which represents the most investigated hormone released from the pineal gland [9], and cannabinoid agents [10] have appeared to inhibit the secretion of both macrophage- related cytokines, including IL-6 and TNF-alpha, and IL-17 released from Th17 lymphocytes. On the other side, Ang 1-7, which is the active product of ACE2 (11), has been proven to play a fundamental anti-inflammatory activity, mainly due to an opposite effect on IL-17-induced inflammatory response and its systemic toxicity [12], including endothelial damage with a consequent enhanced thrombotic predisposition, cardiotoxicity, and acute respiratory distress syndrome (ARDS) [12-14], furtherly amplified by the stimulatory action of IL-17 on IL-6 and TNFalpha production [15]. From this point of view, Covid 19 disease could simply be reinterpreted as due to an acute and severe Ang 1-7 following the block of ACE2 activity induced by the interaction with the viral spike protein. Then, ACE2 does not simply represent the receptor of virus to entry into the cells, but also the molecule, whose block may allow an unregulated exaggerated inflammatory response due an acute Ang 1-7 deficiency [16,17].
Moreover, a diminished ACE2 expression with a consequent Ang 1-7 deficiency has been documented in all inflammatory conditions, including autoimmune diseases [18] and cancer itself [19]. By summarizing, the inflammatory response firstly depends on the functional status of the cytokine network, which in turn is physiologically under a neuroendocrine inhibitory control mainly played by pineal gland-cannabinoid system axis an ACE2- Ang 1-7 system, and the last result of the inflammatory reaction is represented by the local release of inflammatory factors, such as prostaglandins and leukotrienes, with the local production of chemokines as a link between cytokines and local inflammatory factors. Then, at least from a theoretical point of view, it could be possible to control the inflammatory response by acting either on its superior control, represented by the central neuroendocrine regulation of the inflammatory status, or on the local production of inflammatory factors as last event. As far as the pathogenesis of cancer is concerned, it has been shown that cancer-related inflammation, which is mainly mediated by the macrophage system through the release of IL-6 and TNF-alpha, suppresses the anticancer immunity, by representing one of the main causes of cancer progression [20]. Unfortunately, the anti-inflammatory cytokines, including IL-10 and TGF-beta [1,2], may exert a concomitant immunosuppressive action on the anticancer immunity. Then, they cannot be used to counteract cancer-related chronic inflammatory status, because of their immunosuppressive effects. Therefore, it would be more appropriate to modulate cancer-related inflammatory status by acting on its central neuroendocrine regulation, which is mainly exerted by the pineal gland-brain cannabinoid functional unity and ACE2-Ang 1-7 system, and which consists of inhibition of IL- 17 secretion and its negative effects, including cardiotoxicity and endothelial damage responsible for both thrombosis and vascular enhanced permeability. Moreover, the two major anti-inflammatory functional circuits, represented by pineal-cannabinoid axis and ACE2-Ang 1-7 system, are connected by reciprocal interactions. In fact, it has been shown that the pineal hormone MLT [21] and cannabinoids [22] may stimulate ACE2 expression by allowing an enhanced Ag 1-7 production, which in turn has been shown to stimulate cannabinoid receptor expression, by enhancing the endocannabinoid functionless [23]. Ang 1-7 [24] and MLT [25] have also appeared to inhibit TGF-beta secretion, which is one of the main immunosuppressive endogenous agents on the anticancer immunity [2]. Then, the anti-inflammatory activity played by the cytokine network through the release of TGF-beta and IL-10 would concomitantly allow a suppression of the anticancer immunity. On the contrary, the anti-inflammatory action played by the neuroendocrine functional axis constisted of pineal-cannabinoid- Ang 1-7 system is associated with a concomitant stimulation of the anticancer immunity. In addition to their stimulatory action on lymphocyte-dependent anticancer immunity, MLT, as well as other pineal indoles [9,26,27], cannabinoids agonists [28,29] and Ang 1-7 [30-32], have been also appeared to exert a direct anti-proliferative cytotoxic action on cancer cell growth and an anti-angiogenic activity. Preliminary clinical studies have shown the antitumor efficacy of both MLT, either alone [33,34] or in association with cannabinoid agents [10], and Ang 1-7 [35-37],without any important toxicity in the treatment of human neoplasms, including the advanced neoplastic diseases, for whom no other standard effective anticancer therapy was available.
Moreover, both MLT [6-8] and Ang 1-7 [38] have been shown to stimulate the anticancer immunity. In more detail, MLT would promote the anticancer immunity by stimulating Th1-dependent IL-2 secretion [7] and inhibiting IL-17 secretion [39], while the major action of Ang 1-7 would consist of the reverse of macrophagemediated immunosuppression [38]. Ang 1-7 has also appeared to exert a radioprotectant action [35]. Finally, both MLT [7,27,34] and Ang 1-7 [35-38] have appeared to exert important hematopoietic effects, mainly consisting of the stimulation of lymphocyte and platelet generation, with a possible normalization of cancer-related lymphocytopenia and thrombocytopenia, for whom very few other effective drugs may be available. Unfortunately, at present there is no clinical study carried out to evaluate the therapeutic efficacy of a concomitant association between MLT and Ang 1-7 in the treatment of advanced cancer patients eligible for the only best supportive care alone. On these bases, a preliminary phase II study of MLT plus 5-MTT and CBD in association with Ang 1-7 in a group of untreatable advanced cancer patients, for whom no other standard effective anticancer therapy was available.
Patients and Methods
The study included 14 consecutive patients (M/F: 5/9; median age: 63 years, range 46-71). Eligibility criteria were, as follows: histologically proven neoplastic disease; measurable lesions, no double tumor, no availability of other conventional anticancer therapies because of progression under the previous treatments, including chemotherapy, endocrine therapy, targeted-therapy and immunotherapy, depending on the histotype of tumour. After the approval of the Ethical Committee, the experimental protocol was explained to each patient, and written consent was obtained. Tumor histotype were, as follows: brain glioblastoma (GMB):4, lung adenocarcinoma: 2, colorectal cancer:2, breast cancer:2 (triple negative:1), neuroendocrine tumour: 1, renal cell cancer: 1, pharynx carcinoma: 1, endometrial adenocarcinoma:1. Distant organ metastases were present in 10/14 patients (nodes: 1, bone: 1, lung: 3, lung + liver: 1, liver: 2: brain: 2). According to previous clinical studies (10, 34), MLT was given during the dark period of the day half-hour prior to sleep at a dose of 100 mg/day, 5-MTT during the period of maximum light at 10 mg/day, and CBD at 10 mg twice/day (8 AM and 8 PM). All drugs were given orally. Finally, Ang 1-7 was given orally at 0.5 mg twice/day (8 AM and 8 PM) in gastro-protected capsules. We decided to use Ang 1-7 at low-doses because of the possible enhancement of its biological efficacy in association with MLT, which has been proven to interact with ACE2-Ang 1-7 system [21]. The treatment was continued without interruption until the progression of disease. The clinical response was evaluated according to WHO criteria by repeating the radiological examinations, including CT, NMR and PET, at 3-month intervals. Patients were considered as evaluable when they were treated for at least 3 months. Moreover, the immunoinflammatory response was evaluated by analysing changes in lymphocyte-tomonocyte ratio (LMR) values, whose increase has been proven to predict a better prognosis in the advanced neoplastic diseases [40]. Normal values observed in our laboratory (95% confidence limits) were greater than 2.1. Data were statistically analysed by the chisquare test and the Student’s t test, as appropriate.
Results
The clinical characteristics of patients and their clinical response to therapy are reported in Table 1. No complete response was achieved. A partial response (PR) was obtained in 2/14 (14%) patients (GBM: 1; pharynx cancer: 1). A stable disease (SD) occurred in 8/14 (57%) patients (GBM: 1, colon cancer:2, lung cancer: 1, neuroendocrine tumours:1; renal cell cancer: 1, breast cancer: 1, endometrial cancer: 1). Therefore, a disease control (PR + SD) was achieved in 10/14 (71%), whereas the remaining 4/14 (29%) had a progressive disease (PD). The median duration of response was 5 months (range 3- 10+). An abnormally low values of LMR prior to therapy was found in 6/14 (43%). A normalization of LMR values, due to both increase in lymphocyte count and decrease in monocyte number, was obtained on therapy in 4/6 (67%) patients, who obtained a SD, whereas the other two patients had a PD. In addition, lymphocyte mean number observed on therapy in patients who achieved PR or SD was significantly higher than in patients who had a PD (1648 +/- 115 vs 1024 +/- 188 /mm3, P< 0.05). Moreover, a clear relief of asthenia was obtained in 8/10 (80%) patients with important asthenic symptomatology prior to study. Not only, but the relief of asthenia was referred to be associated with a perception of a major integration between consciousness and biological body. Finally, 9/14 (64%) patients referred an increase in diuresis values. No toxicity occurred on study, and in particular no important decline in blood pressure values was observed.
Table 1: Characteristics of patients and their response to therapy.
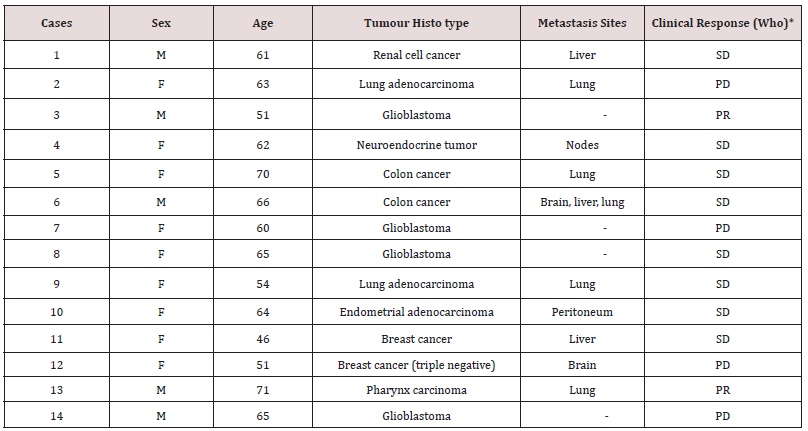
*PR: partial response; SD: stable disease; PD: progressive disease.
Discussion
The results of this preliminary study furtherly confirm previous clinical investigations, which had already shown that advanced cancer patients eligible for the only palliative therapy may achieved a disease control in a considerable number of cases through the administration of some natural endogenous anticancer molecules, the most known of them is represented by the pineal MLT [33,34], without any toxicity. Moreover, this study would suggest that the administration of Ang 1-7 may furtherly enhance the antitumor efficacy of high-dose MLT therapy alone, as observed in previous clinical investigations [34]. Then, the results of this study would justify successive randomized clinical studies with high-dose MLT alone versus high-dose MLT plus Ang 1-7 in the treatment of patients with disseminated cancer. Moreover, it needs to be remarked that in the present study Ang 1-7 was employed at low doses. Then, since Ang 1-7 anticancer efficacy has appeared to be a dose-dependent phenomenon [37,38], more interesting results could by achieved by using high-dose Ang 1-7. In addition, from a palliative point of view, this study seems to show a particular efficacy of Ang 1-7 in the treatment of asthenia, since no clear efficacy in the treatment of asthenia was previously described with high-dose MLT alone [34]. This evidence is important from both therapeutic and pathological points of view, since no standard therapy of asthenia is available, and the neurochemical mechanisms responsible for the asthenia as a failure of forces need to be better investigated and understood, obviously in the case that asthenia is not due to an important anaemia. Recently, it has been shown the existence of an ACE2-Ang 1-7 system also at brain level, which would be involved in influencing the cognitive functions and other psychological profiles [10,11,16]. Therefore, the apparent efficacy of Ang 1-7 in the treatment of cancer-related asthenia would suggest that Ang 1-7 may play a physiological psychic function in influencing the perception of own forces and well-being. In conclusion, according to these results, the idea of a palliative therapy alone would have to be abrogated in the medical Oncology, and substituted by potential therapeutic neuroimmune regimens, consisting of the administration of the same endogenous molecules of the human body provided by a documented anticancer non-toxic activity, in particular the pineal hormone MLT and Ang 1-7 itself, as shown by the present study.
References
- Dennis KL, Blatner NR, Gounari F, Khazaie K (2013) Current status of IL-10 and regulatory T cells in cancer. CurrOpin Oncol 25: 637-645.
- Yang L, Pang Y, Moses HL (2010) TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 31: 220-227.
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA (1982) Lymphokine-activated killer cell phenomenon. J Exp Med 155: 1823-1841.
- Banks RE, Patel PM, Selby PJ(1995) Interleukin-12: a new clinical player in cytokine therapy. Br J Cancer 71: 655-659.
- Lissoni P (2017) Therapy implications of the role of interleukin-2 in cancer. Exp Rev Clin Immunol 13: 491-498.
- Regelson W, Pierpaoli W (1987) Melatonin: a rediscovered antitumor hormone? Cancer Invest 5: 379-385.
- Maestroni GJM (1993) The immune neuroendocrine role of melatonin. J Pineal Res 14: 1-10.
- Lissoni P (1999) The pineal gland as a central regulator of cytokine network. Neuroendocrinol Lett 20: 343-349.
- Reiter RJ (2004) Mechanisms of cancer inhibition by melatonin. J Pineal Res 37: 213-214.
- Lissoni P, Rovelli F, Messina G, Monzon A, Pensato S, et al. (2019) A review on the neuroendocrine regulation of cytokine secretion: possible modulation of the cytokine network by the pineal hormone melatonin and cannabidiol. Oncol Res Rev 2: 1-4.
- Capettini LS, Montecucco F, Mach F, Stergiopulos N, Santos RA, da Silva RF (2012) Role of renin-angiotensin system in inflammation, immunity,and aging. Curr Pharm Des 18: 963-970.
- Simoes-e-Silva AC, Silveira KD, Ferreira AJ, Teixeira MM (2013) ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol 169: 477-492.
- Robert M, Miossec P (2017) Effects of interleukin-17 on the cardiovascular system. Autoimmune Rev 16: 984-991.
- Li Q, Gu Y, Tu Q, Wang K, Gu X, Ren T (2015) Blockade of interleukin-17 restrains the development of acute lung injury. Scand J Immunol 83(3):203-11.
- Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, et al. (2007) Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol 179: 1423-1426.
- Verdecchia P, Cavallini C, Spanevello A, Angeli F (2020) The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Int Med 76: 14-20.
- Peirò C, Moncada S (2020) Substituting angiotensin-(1-7) to prevent lung damage in SARS-CoV-2 infection? Circulation 141: 1665-1666.
- Kawajiri M, Mogi M, Higaki N, Matsuota T, Ohyagi Y, et al.(2019) Angiotensin-converting enzyme (ACE) and ACE2 levels in the cerebrosopinal fluid in patients with multiple sclerosis. Mult Scler 15: 262-265.
- Gallagher PE, Arter AL, Deng G, Tallant EA (2014) Angiotensin-(1-7): a peptide with anti-cancer activity. Curr Med Chem 21: 2417-2423.
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-relatedinflammation. Nature 454: 436-444.
- Campos LA, Cipolla-Neto J, Amaral FG, Michelini LC, Badeer M, Baltatu OC (2013) The angiotensin-melatonin axis. Int J Hypertension.
- Sainz-Cort A, Heeroma JH (2020) The interaction between the endocannabinoid system and the renin-angiotensin system and its potential implication for Covid-19 infection. J Cannabis Res 2: p.23.
- Brosnihan KB, Pulgar VM, Gallagher PE, Neves LAA, Yamaleyeva LM (2015) Local uterine Ang-(1-7) infusion augments the expression of cannabinoid receptors and differentially alters endocannabinoid metabolizing enzymes in the decidualized uterus of pseudo-pregnant rats. ReprodBiol Endocrinol13: p.5.
- Cook KL, Metheny Barlow LJ, Tallant EA, Gallagher PE (2010) Angiotensin-(1-7) reduced fibrosis in orthotopic breast tumors. Cancer Res 70: 8319-8328.
- Wang YR, Hong RT, Xie YY, Xu JM (2018) Melatonin ameliorates liver fibrosis induced by carbon tetrachloride in rats via inhibiting TGF-beta 1/Smadsignaling. Curr Med Sci 38: 236-244.
- Sze SF, Ng TB, Liu WK (1993) Antiproliferative effect of pineal indoles on cultured tumor cell lines. J Pineal Res 14: 27-33.
- Lissoni P, Messina G, Rovelli F (2012) Cancer as the main aging factor for humans and reversal of cancer-induced aging processes on metabolic and immune reactions by the pineal hormones other than melatonin: the fundamental role of 5-methoxytryptamine. Curr Aging Sci 5: 231-235.
- Grotenhermen F (2004) Pharmacology of cannabinoids. Neuroendocrinol Lett 25: 14-23.
- Nagarkatti P, Pandey R, Rieder SA, Hedge VL, Nagarkatti M (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1: 1333-1349.
- Gallagher PE, Tallant EA (2004) Inhibition of human lung cancer cell growth by angiotensin-(1-7). Carcinogenesis 25: 2045-2452.
- Feng Y, Ni L, Wan H, Fan L, Fei X, et al. (2011) Overexpression of ACE2 produces antitumor effects via inhibition of angiogenesis and tumor cell invasion in vivo and in vitro. Oncol Re 26: 1157-1164.
- Souza-Santos RA (2019) The role of angiotensin-(1-7) in cancer. Angiotensin-(1-7) 22: 219-229.
- Mills E, Wu P, Seely D, Guyatt G (2005) Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J Pineal Res 39: 360-366.
- Lissoni P, Rovelli F, Brivio F, Messina G, Lissoni A, et al. (2018) Five year-survivalwith high-dose melatonin and other antitumor pineal hormones in advanced cancer patients eligible for the only palliative therapy. Res J Oncol 2: 1-7.
- Rodgers KE, Xiong S, Di Zerega GS (2002) Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother Pharmacol 49: 403-411.
- Krishnan B, Torti FM, Gallagher PE, Tallant EA (2013) Angiotensin-(1-7) reduces proliferation and angiogenesis of human prostate cancer xenografts with a decrease in angiogenic factors and an increase in sFlt-1. Prostate 73: 60-70.
- Pham H, Schwartz BM, Delmore JE, Reed E, Cruickshank S, et al. (2013) Pharmacodynamic stimulation of thrombogenesis by angiotensin-(1-7) in recurrent ovarian cancer patients receiving gemcitabine and platinum-based chemotherapy. Cancer Chemother Pharmacol 71: 965-972.
- Rodgers KE, Oliver J, diZerega GS (2006) Phase I/II dose escalation study of angiotensin 1-7 administered before and after chemotherapy in patients with newly diagnosed breast cancer. Cancer Chemother Pharmacol 57: 559-568.
- Kuklina EM, Glebezdina NS, Nekrasova IV (2016) Role of melatonin in the regulation of differentiation of T cells producing interleukin-17 (Th17). Bull Exp Biol Med 160: 656-658.
- Gu L, Li H, Chen L, Ma X, Li X, et al. (2016) Prognostic role of lymphocyte-to-monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget 3: 7876-7881.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

.png)


