Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1644
Review articleOpen Access
Role of Carbon Mono-Oxide (CO) Signaling During Trophoblast Invasion in Humans Volume 2 - Issue 2
Ruby Dhar, Indrani Mukherjee, Sunil Singh, Sandep Goswami and Subhradip Karmakar*
- Ruby Dhar, Indrani Mukherjee, Sunil Singh, Sandep Goswami and Subhradip Karmakar*
Received: January 08, 2019; Published: January 17, 2019
Corresponding author: Subhradip Karmakar, Department of Biochemistry, All India Institute of Medical Sciences, New Delhi, India
DOI: 10.32474/OAJRSD.2019.02.000133
Abstract
Trophoblast cells originating from the outer tropho ectodermal layer of the blastocyst forms the placenta, a transient organ that is instrumental in supporting pregnancy. The process begins with the interaction between two genetically dissimilar tissues, with the trophoblast cells, like a parasite programing the maternal system to suit its survival. A successful pregnancy is multistep process initiating with the process of proper orientation and apposition of the floating blastocyst to the maternal uterine endometrium. This is followed by the pseudo malignant trophoblasts invading the maternal decidua and securing an adequate supply of oxygen and nutrient to the fetus. Several cytokines and hormones are known to orchestrate this process derailment of which often have far reaching consequences. Carbon monoxide (CO) originating from HMOX (Heme Oxygenase) system is believed to play a crucial role in this process. Though not much is known about this system, preliminary findings from our lab points out an important role of this gaseous molecule during trophoblast invasion.
Abbrevations:CO: Carbon Monoxide; HMOX: Heme Oxygenase; MMP: Matrix Metalloproteinases; uPA: Urokinase type Plasminogen Activator; NO: Nitric Oxide; NOS: Nitric Oxide Synthase; MMP: Matrix Metallo Protease; ECM: Extracellular Matrix; EVTs: Extra Villous Cytotrophoblast; MAPK: Mitogen Activated Protein Kinase; PE: Preeclampsia; HTN: Hypertensiong
Introduction
Successful implantation is an outcome between two genetically dissimilar tissues that often depends on synchronization between the developmental stages of the embryo and the molecular events that are induced by paracrine and autocrine regulators [1]. The process of implantation begins six to seven days following fertilization [2,3] and consists basically of three stages [4].
Stage1: Apposition: It is the first stage denoting the initial, still unstable, adhesion of the blastocyst to the uterine wall. Micro protrusions from the apical uterine epithelium surface also known as Pino pods, inter-digitate with the microvilli on the apical surface of the multinucleated syncytiotrophoblast [5].
Stage2: Adhesion: During this process establishment of an increased physical interaction is established between the blastocyst and the uterine epithelium.
Stage 3: Invasion: Perhaps one of the most crucial process that aims to anchor the developing embryo into the uterine environment beginning with the penetration of the multinucleated syncytiotrophoblasts through the uterine endometrium followed by invasion of the cytotrophoblasts upto the inner third of the myometrium and terminating with remodeling of the maternal spiral arteries [6].
While floating villi remains bathed in maternal blood, villous cytotrophoblast cells originating at the tip of the anchoring villi proliferate outwards from the underlying basement membrane to form trophoblast cell columns from which cells migrate into the decidua (interstitial trophoblast cells) and invade the maternal spiral arteries and (endovascular trophoblast cells). The latter allows the trophoblasts to be in direct contact with the maternal blood establishing the uteroplacental circulation [7]. Trophoblast invasion and migration is probably controlled by components of the trophoblast itself and maternal microenvironment, through molecular and cellular interactions. For successful invasion to occur, extra villous emerging out from the trophoblast cell column trophoblast must perform a range of functions; transformation of the maternal spiral arteries, tolerate hypoxia, proliferate and die by apoptosis (programmed cell death), differentiate, adhere to and digest the extracellular matrix, move and interact with the maternal immune system [8]. Each of these functions has multiple overlapping control systems so that trophoblast invasion is a finely controlled balance of competing mechanisms.
Trophoblast cells are unique to display pseudo malignant properties by virtue of which they can invade the maternal uterus and invade into the surrounding tissue. This capability is widely associated with tumors, and, indeed, the invasive behavior of both is rather similar. The sticking difference is that trophoblast cell invasion is temporally and locally controlled in contrast to unlimited and uncontrolled tumor invasion. It initiates immediately after embryo implantation into the endometrium. Like invasive tumors, trophoblasts remodel the ECM by secretion of matrix degrading proteases, such as matrix metalloproteinases (MMP), uPA (Urokinase type plasminogen activator), cathepsins etc. which efficiently degrades the extracellular matrix and the surrounding tissue. Thereby, these proteases prepare and allow true invasion of trophoblasts. The invasive capacities of trophoblasts are positively and negatively regulated by numerous cytokines including IL1beta, TGFbeta 1 as well as several cell and ECM proteins [9,10], LIF [11] hepatocyte growth factor, granulocyte macrophage-colony stimulating factor and others. These cytokines interact via specific cell surface receptors with the trophoblast cells, in which they activate diverse intracellular signaling cascades like MAPK [12], Janus kinase/signal transducers [13], PI3 kinase [14] and activators of transcription (STAT) pathway [15,16]. Especially phosphorylated STAT3 enhances invasiveness of tumors and trophoblast cells, where it is mainly activated by LIF [17]. The balance between different intracellular molecules seems to be a key regulator of tumor and trophoblast invasion [18,19].
CO as Signaling Molecule
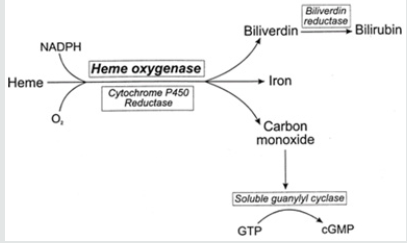
Apart from cytokines, new evidence shows that gaseous molecules like NO and CO might also play an important role in trophoblast invasion. Nitric oxide (NO) is a multifunctional signaling molecule and might play a role in human reproduction [20]. NO is generated from L-arginine by the catalytic action of the enzyme, nitric oxide synthase (NOS) [21]. In recent years, enthusiasm has emerged for a possible role of CO as bonafide signaling molecular. Heme oxygenase-1 (HO-1) catalyzes the first and rate-limiting step in heme catabolism toward biliverdin, carbon monoxide (CO), and free iron [22]. HO-1 represents the stress-responsive HO isoform and is encoded by the Hmox1 gene. As shown in vitro and in vivo studies, HO-1 is cytoprotective and exerts anti-inflammatory effects, while regulating cell proliferation [23-26] prevents tissue injury; but also, modulates innate and adaptive immune responses [27]. Thus, HO-1 is a central player in suppressing the pathogenesis of immune-mediated inflammatory diseases [28]. The exogenous application of CO by inhalation can restore the cytoprotective effects HO-1 [29,30-32]. Hence, CO mediates, to a large extent, the salutary effects of HO-1. However, other products of heme catabolism such as iron and biliverdin, might act in a similar manner. Heme oxygenase’s exist primarily in two forms, one constitutive (HO-2) and inducible (HO-1) isoforms. While HO-2 has distinct tissue localization, being predominantly expressed in testes, brain, and the endothelium, HO-1 is upregulated in all tissues examined following several kinds of stress stimuli, including oxidative stress, which is an underlying factor in different pathological states [33]. In addition to CO, heme catabolism generates ferrous iron (Fe2+) and biliverdin, an aqueous tetrapyrrolic pigment that is then reduced to bilirubin by biliverdin reductase [34] (Figure 1).
Trophoblast Invasion: Role of MMPs
MMP (Matrix metallo protease) are the family of proteases that includes over 20 zinc-dependent enzymes sharimng common functional domains. These enzymes were initially characterized by their extensive ability to degrade extracellular matrix proteins including collagens, laminin, fibronectin, vitronectin, aggrecan and proteoglycans [35]. Recent studies further demonstrate that MMPs cleave many other types of peptides and proteins independent of proteolytic activity [36]. MMPs and their natural tissue inhibitors TIMPs are crucial in coordinated breakdown and remodeling of the extracellular matrix (ECM) in physiological and pathological situations. Placenta formation during pregnancy involves the participation of MMPs/TIMPs system that plays important roles in regulating the extravillous cytotrophoblast (EVTs) invasion. Interstitial trophoblast originating from trophoblast cell column that finally remodel maternal endometrium. Matrix metalloproteinases (MMPs), play a crucial role in matrix proteolysis and remodeling of the basement membrane. Secretion of MMPs, is involved in the normal invasion process as well as in the invasive character of tumor cells and metastasis formation [37,38].
MMPs are a family of zinc and calcium-dependent proteolytic enzymes, which should be activated by prodomain proteolytic cleavage, or the alteration of its structure [39]. Both pro-MMPs and active MMPs may be inhibited by metalloproteinase tissue inhibitors (TIMPs) that are secreted by MMPs [40]. Thus, the invasive character of cells is determined by the protease/inhibitor ratio [41]. Analyses of MMP and TIMP secretion and activity in trophoblast and trophoblast-derived cells point out the role of gelatinase-A (MMP-9) and gelatinase-B (MMP-2) in the high invasive capacity of first trimester trophoblasts and support the role of metalloproteinases 2 and 9 during pregnancy [42,43]. However, MMP expression patterns may be different between normal trophoblasts and choriocarcinoma cells [44] and may vary between early and late trophoblasts [45,46].
Regulation of MMPs
Accumulating data from various studies strongly suggest that MMPs are tightly regulated, starting from the level of gene expression all the way to zymogen activation and endogenous inhibition, with each level controlled by multiple factors. Several recent findings indicate that cell-ECM and cell-cell interactions contribute an additional layer of regulation at all levels, and thus matrix and basement membrane offer secondary levels of regulation. The tissue inhibitors of metalloproteinases or TIMPs consist of a small family of four homologous and low molecular weight proteins and were found to suppress MMP activity by complexing in a definite molar stoichiometry thereby preventing extracellular matrix turnover during tissue remodeling. TIMP concentrations generally far exceed the concentration of MMPs in tissue and extracellular fluids, thereby limiting their proteolytic activity to focal pericellular sites [47,48]. TIMPs are originally known to inhibit the MMP activities. TIMP is downregulated or repressed in a variety of cancer. Epigenetic modification and promoter hypermethylation are believed to be responsible for TIMP silencing. Besides orchestrating invasion, MMPs also regulates additional events that supports tumor survival and metastasis like tumor promotion, angiogenesis and the establishment and growth of metastatic lesions in distant organ sites. The signaling pathways that lead to activation of MMPs are not clearly know and are believed to be multifactorial. Multiple hormones, cytokines and growth factors can induce MMP expression [49-52] although the tissue specificity of the diverse family members is mainly achieved by the combination of different transcriptional control mechanisms [53,54]. The integration of multiple signaling pathways, coupled with the cooperation between several cis-regulatory elements found at the MMP promoters facilitates the strict spatiotemporal control of MMP transcriptional activity.
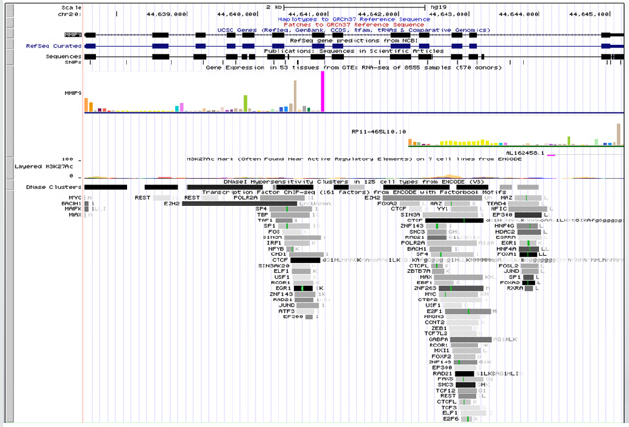
TF binding at MMP2 (Figure 2) and MMP9 (Figure 3)
Figure 2: .Upstream and downstream regulators of MMP-2 (72kDa gelatinase) gene as revealed from ENCODE.
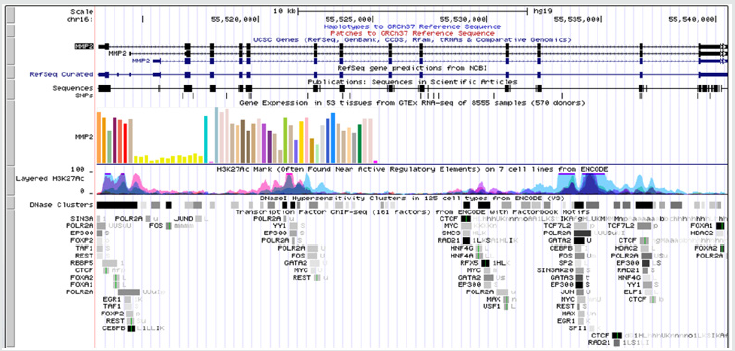
Figure 3: Upstream and downstream regulators of MMP9 (92kDa gelatinase) gene as revealed from ENCODE.
Involvement of CO
CO seems to regulate vascular processes such as vessel tone, smooth muscle proliferation and platelet aggregation. Much of these effects of CO depend on the activation of guanylate cyclase system by direct binding to the heme moiety of the enzyme, stimulating the production of cyclic 3’:5’-guanosine monophosphate [55]. CO also exerts novel anti-inflammatory and anti-apoptotic effects dependent through involvement of the p38 mitogen activated protein kinase (MAPK)-signaling pathway. One target of CO appears to be the L-type Ca2+ channel [56,57]; CO inhibits recombinant and native forms of this cardiac channel via mitochondria-derived ROS, which also contributes to the protective effects of CO.
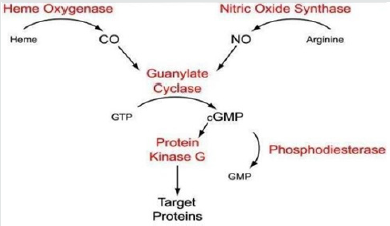
CO and NO Signaling
Figure 4 CO is shown to activate calcium dependent potassium channels as well as MAPK pathways with special focus on p38 family of MAPK [58]. This is mostly in response to stress and inflammation. CO signaling is also associated with apoptosis, proliferation and invasion as well as activation of several heat shock proteins like HSP70, 90 and caveolin [58,59]. Evidence also suggest a cross talk between CO signaling and NFkb activation [60,61]. Our in-silico analysis showed several of these effectors to be potentially recruited to upstream elements of MMPs. It is thus quite conceivable that upstream CO signaling cumulating to MMPs activation or repression.
CO and Preeclampsia
Preeclampsia (PE) is a serious condition of pregnancy which presents as proteinuria and hypertension (HTN) [62] and is associated with underlying maternal vascular dysfunction [62,63]. There is no known cure other than delivery of the feto-placental unit. PE is thought to originate in the placenta, as a result of impaired implantation, leading to poor placental perfusion and hypoxia [64]. The resulting ischemia increases placental apoptosis and subsequent shedding of placental debris [65,66].
Conclusion
Carbon monoxide (CO) is generated as an industrial and house hold pollutant during incomplete combustion of carbon-containing compounds. It is believed to be toxic with acute and chronic serious complications. In addition to exogenous sources, CO is also produced endogenously by the activity of heme oxygenase’s (HOs) system and the physiological significance of HO-derived CO has only recently elucidated. CO exerts a multitude of effect like vasoactive, anti-proliferative, anti-oxidant, anti-inflammatory and anti-apoptotic effects and contributes substantially to the important role of the inducible isoform HO-1 as a mediator of tissue protection and host defense. Further CO signaling was found to be integrated with MAPK, NFkB and with other effectors that in turn can influence MMP production and function. It is thus reasonable to conclude that a dysregulated HMOX axis can perturb MMPs and therefore trophoblastic invasion. Thus, a component of CO signaling can undoubtedly contribute to pathogenesis of eclampsia. It needs further research to decipher if CO acts alone or in conjugation with NO due to the overlap between these pathways. In addition to its effect on MMPs, CO through its capacity to engage NFkb pathway could also orchestrate the immune microenvironment of the placenta thereby regulating the feto-maternal cross talks and recognition, perturbation of which contributes to pregnancy failure and fetal loss. CO is thus emerging as a pleiotropic modulator in feto-maternal biology with clinical
References
- Staun-Ram E, Shalev E (2005) Human trophoblast function during the implantation process. Reprod Biol Endocrinol 20(3): 56.
- Bischof P, Campana A (2000) Molecular mediators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol 14(5): 801-814.
- Vigano P, Mangioni S, Pompei F, Chiodo I (2003) Maternal-conceptus Cross Talk - A Review. Placenta 24: S56-S61.
- Norwitz ER, Schust DJ, Fisher SJ (2001) Implantation and the survival of early pregnancy. N Engl J Med 345(19): 1400-1408.
- Lopata A, Bentin-Ley U, Enders A (2002) “Pinopodes” and Implantation. Reviews in Endocrine metabolic Disorders 3(2): 77-86.
- Pijnenborg R (1988) Establishment of uteroplacental circulation. Reprod Nutr Dev 28(6B): 1581-1586.
- Soundararajan R, Rao AJ (2004) Trophoblast ‘pseudo-tumorigenesis’: significance and contributory factors. Reprod Biol Endocrinol 25(2): 15.
- Carlos Salomon, Sarah W Yee, Murray D Mitchell, Gregory E Rice (2014) The Possible Role of Extravillous Trophoblast-Derived Exosomes on the Uterine Spiral Arterial Remodeling under Both Normal and Pathological Conditions. BioMed Research International p. 10.
- Karmakar S, Das C (2002) Regulation of trophoblast invasion by IL- 1beta and TGF-beta1. Am J Reprod Immunol 48(4): 210-219.
- Karmakar S, Das C (2004) Modulation of ezrin and E-cadherin expression by IL-1betaand TGF-beta1 in human trophoblasts. J Reprod Immunol 64(1-2): 9-29.
- Pereira de Sousa FL, Chaiwangyen W, Morales-Prieto DM, Ospina-Prieto S, Weber M, et al. (2017) Involvement of STAT1 in proliferation and invasiveness of trophoblastic cells. Reprod Biol 17(3): 218-224.
- Malik A, Pal R, Gupta SK (2017) Interdependence of JAK-STAT and MAPK signaling pathways during EGF-mediated HTR-8/SVneo cell invasion. PLoS One 12(5): e0178269.
- Xu C, Li X, Guo P, Wang J (2017) Hypoxia-Induced Activation of JAK/ STAT3 Signaling Pathway Promotes Trophoblast Cell Viability and Angiogenesis in Preeclampsia. MedSci Monit 23: 4909-4917.
- Che G, Wang Y, Zhou B, Gao L, Wang T, et al. (2018) Knockdown of Heparanase Suppresses Invasion of Human Trophoblasts by Activating p38 MAPK SignalingPathway. Dis Markers 2018.
- Banerjee P, Malik A, Malhotra SS, Gupta SK (2019) Role of STAT signaling and autocrine action of chemokines during H(2) O (2) induced HTR-8/ SVneo trophoblastic cells invasion. J Cell Physiol 234(2): 1380-1397.
- Borg AJ, Yong HE, Lappas M, Degrelle SA, Keogh RJ, et al. (2015) Decreased STAT3 in human idiopathic fetal growth restriction contributes to trophoblast dysfunction. Reproduction 149(5): 523-532.
- Sousa FL, Morales Prieto DM, Ospina Prieto S, Chaiwangyen W, Daher S, et al. (2012) PP007. Effects of STAT1 suppression on ERK1/2 in trophoblastic cells. Pregnancy Hypertens 2(3): 243.
- Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR (2008) Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3). Hum Reprod Update 14(4): 335-344.
- Fitzgerald JS, Busch S, Wengenmayer T, Foerster K, de la Motte T, et al. (2005) Signal transduction in trophoblast invasion. Chem Immunol Allergy 88: 181-199.
- Edwards TM, Hamlin HJ (2018) Reproductive endocrinology of environmental nitrate. Gen Comp Endocrinol 265: 31-40.
- Díaz-Pérez F, Radojkovic C, Aguilera V, Veas C, González M, et al. (2012) L-arginine transport and nitric oxide synthesis in human endothelial progenitor cells. J Cardiovasc Pharmacol 60(5): 439-449.
- Tenhunen R, Marver HS, Schmid R (1968) The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 61(2): 748-755.
- Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, et al. (1998) Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med 4(9): 1073-1037.
- Otterbein LE, Choi AM (2000) Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 279(6): L1029-1037.
- Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, et al. (2000) Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 192(7): 1015-1026.
- Duckers HJ, Boehm M, True AL, Yet SF, San H, et al. (2001) Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med 7(6): 693-698.
- Duckers HJ, Boehm M, True AL, Yet SF, San H, et al. (2001) Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med 7(6): 693-698.
- Soares MP, Marguti I, Cunha A, Larsen R (2009) Immunoregulatory effects of HO-1: how does it work? Curr Opin Pharmacol 9(4): 482-489.
- Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, et al. (2000) Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 192(7): 1015-1026.
- Alam J, Wicks C, Stewart D, Gong P, Touchard C, et al. (2000) Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem 275(36): 27694-27702.
- Ryter SW, Otterbein LE, Morse D, Choi AM (2002) Heme oxygenase/ carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem 234-235(1-2): 249-263.
- Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 73(17): 3221-3247.
- Van Dijk R, Aronson SJ, de Waart DR, van de Graaf SF, Duijst S, et al. (2017) Biliverdin Reductase inhibitors did not improve severe unconjugated hyperbilirubinemia in vivo. Sci Rep 7(1): 1646.
- Shang L, Rockwell NC, Martin SS, Lagarias JC (2010) Biliverdin amides reveal roles for propionate side chains in bilin reductase recognition and in holophytochrome assembly and photoconversion. Biochemistry 49(29): 6070-6082.
- Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123(Pt 24): 4195-4200.
- Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463-516.
- Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141(1): 52-67.
- Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N (2000) Metalloproteinases: Role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res 2(4): 252-257.
- Kim YS, Joh TH (2012) Matrix metalloproteinases, new insights into the understanding of neurodegenerative disorders. Biomol Ther (Seoul) 20(2): 133-143.
- Brew K, Nagase H (2010) The tissue inhibitors of metalloproteinases (TIMPs): Anancient family with structural and functional diversity. Biochim Biophys Acta 1803(1): 55-71.
- Chénais B (2012) Matrix metalloproteinase-2 and -9 secretion by the human JAR choriocarcinoma cell line is stimulated by TNF-α. Advances in Bioscience and Biotechnology 3: 51-56.
- Kizaki K, Ushizawa K, Takahashi T, Yamada O, Todoroki J, et al. (2008) Gelatinase (MMP-2 and -9) expression profiles during gestation in the bovine endometrium. Reprod Biol Endocrinol 6: 66.
- Espino Y Sosa S, Flores-Pliego A, Espejel-Nuñez A, Medina-Bastidas D, Vadillo-Ortega F, et al. (2017) New Insights into the Role of Matrix Metalloproteinases in Preeclampsia. Int J Mol Sci 18(7): E1448.
- Jelena Anacker, Sabine E Segerer, Carsten Hagemann, Sonja Feix, Michaela Kapp, et al. (2011) Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases, MHR: Basic science of reproductive medicine 17(10): 637-652.
- Cohen M, Wuillemin C, Irion O, Bischof P (2010) Role of decidua in trophoblastic invasion. Neuro Endocrinol Lett 31(2): 193-197
- Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, et al. (2001) Human trophoblast invasion and spiral artery transformation: The role of PECAM-1 in normal pregnancy, preeclampsia and fetal growth restriction. Am J Pathol 158(5): 1713-1721.
- Basak S, Sarkar A, Mathapati S, Duttaroy AK (2018) Cellular growth and tube formation of HTR8/SVneo trophoblast: Effects of exogenously added fatty acid-binding protein-4 and its inhibitor. Mol Cell Biochem 437(1-2): 55-64.
- Rahat B, Sharma R, Bagga R, Hamid A, Kaur J (2016) Imbalance between matrix metalloproteinases and their tissue inhibitors in preeclampsia and gestational trophoblastic diseases. Reproduction 152(1): 11-22.
- Yan C, Boyd DD (2007) Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211(1): 19-26.
- Reuben PM, Cheung HS (2006) Regulation of matrix metalloproteinase (MMP) gene expression by protein kinases. Front Biosci 11: 1199-1215.
- Clark IM, Swingler TE, Young DA (2007) Acetylation in the regulation of metalloproteinase and tissue inhibitor of metalloproteinases gene expression. Front Biosci 12: 528-535.
- Jiang XF, Ding L, Tian Y, Han N, Li ZQ (2018) Interaction of STAT3 and RelB modulates MMP-1 in colon cancer. Chem Biol Interact 293: 94-99.
- Yu LJ, Wang B, Parobchak N, Roche N, Rosen T (2015) STAT3 cooperates with the non-canonical NF-κB signaling to regulate pro-labor genes in the human placenta. Placenta 36(5): 581-586.
- Wang W, Yang C, Wang XY, Zhou LY, Lao GJ, et al. (2018) MicroRNA-129 and -335 Promote Diabetic Wound Healing by Inhibiting Sp1-Mediated MMP-9 Expression. Diabetes 67(8): 1627-1638.
- Li Y, Lorca RA, Ma X, Rhodes A, England SK (2014) BK channels regulate myometrial contraction by modulating nuclear translocation of NF-κB. Endocrinology 155(8): 3112-3122.
- Striessnig J, Ortner NJ, Pinggera A (2015) Pharmacology of L-type Calcium Channels: Novel Drugs for Old Targets? Curr Mol Pharmacol 8(2): 110-122.
- Duckles H, Boycott HE, Al-Owais MM, Elies J, Johnson E, et al. (2015) Heme oxygenase-1 regulates cell proliferation via carbon monoxidemediated inhibition of T-type Ca2+ channels. Pflugers Arch 467(2): 415- 427.
- Leffler CW, Parfenova H, Jaggar JH (2011) Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol 301(1): H1-H11.
- Bełtowski J, Jamroz A, Borkowska E (2004) Heme oxygenase and carbon monoxide in the physiology and pathology of the cardiovascular system. Postepy Hig Med Dosw 58: 83-99.
- Kim HS, Loughran PA, Rao J, Billiar TR, Zuckerbraun BS (2008) Carbon monoxide activates NF-kappaB via ROS generation and Akt pathways to protect against cell death of hepatocytes. Am J Physiol Gastrointest Liver Physiol 295(1): G146-G152.
- Chhikara M, Wang S, Kern SJ, Ferreyra GA, Barb JJ, et al. (2009) Carbon Monoxide Blocks Lipopolysaccharide-Induced Gene Expression by Interfering with Proximal TLR4 to NF-κB Signal Transduction in Human Monocytes. PLoS ONE 4(12): e8139.
- Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM (2011) Preeclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 7: 467-474.
- P Brown M, Simpson JM, Davis G (2005) Proteinuria in pre-eclampsia: how much matters? BJOG 112(3): 280-285.
- Powers RW, Jeyabalan A, Clifton RG, Van Dorsten P, Hauth JC, et al. (2010) Soluble fms-Like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. Eunice Kennedy Shriver National Institute of Child Health Human Development Maternal-Fetal Medicine Units Network. PLoS One 5(10): e13263.
- Tossetta G, Fantone S, Giannubilo SR, Marinelli Busilacchi E, Ciavattini A, et al. (2018) Pre-eclampsia onset and SPARC: A possible involvement in placenta development. J Cell Physiol.
- English FA, Kenny LC, McCarthy FP (2015) Risk factors and effective management of preeclampsia. Integr Blood Press Control 8: 7-12.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...