Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1644
Research ArticleOpen Access
Importance of Chronic Endometritis (CE) in RIF-An Update on Diagnosis and Treatment Volume 2 - Issue 5
Kulvinder Kochar Kaur1*, Gautam Allahbadia2 and Mandeep Singh3
- 1Scientific Director, Kulvinder Kaur Centre for human reproduction, India
- 2Department of Obstt &Gynae, Scientific Director Ex-Rotunda-A Centre for Human reproduction, India
- 3Consultant Neurologist, Swami Satyanand Hospital, India
Received: November 05, 2019; Published: November 18, 2019
Corresponding author: Kulvinder Kochar Kaur, Scientific Director, Kulvinder Kaur Centre for human reproduction, India
DOI: 10.32474/OAJRSD.2019.02.000146
Abstract
Chronic Endometritis(CE) refers to continuing inflammation within the endometrial lining that is secondary to bacterial organisms. Reproductive failure has been associated with CE for labelling a diagnosis of plasma cells(PC) visualization within the endometrial stroma is considered the criteria. The prevalence in infertile women especially those with recurrent implantation failure(RIF) varies markedly. This discrepancy is there because of no diagnostic criteria that has been agreed upon. Here we have carried out a systematic review to decide what should be the best diagnostic criteria, either on the basis of EB or hysteroscopy as per the international committee on the hysteroscopy group or the newer data that suggests checking PC density(PCD) i n endometrial biopsy (EB) samples based on presence of PC even in fertile women and using 95% of PCD in fertile women and utilization of next generation sequencing of 16S ribosomal RNA subunit. Further utilization of antibiotics orally has been the conventionally used methods of treating CE. But 2 groups have demonstrated good cure rate of CE following direct instillation of antibiotics/antibiotics with dexamethasone in the uterine cavity that resulted in spontaneous conceptions which suggests intrauterine administration of antibiotics once a diagnosis of CE has been made remains the best answer for RIF patients as seen with spontaneous conceptions though more randomized trials are needed for the same.
Keywords: CE; PCD; RIF; uterine cavity antibiotics/antibiotics with dexamethasone; hysteroscopy; 16S ribosomal RNA subunit
Introduction
Chronic Endometritis (CE) refers to continuing inflammation within the endometrial lining that is secondary to bacterial organisms. Reproductive failure has been associated with CE [1-6]. For labelling a diagnosis of CE, plasma cells visualization within the endometrial stroma is considered the criteria [6]. The prevalence in infertile women especially those with recurrent implantation failure (RIF) varies between 14%-58%[7]. Clinically CE presents with very subtle symptoms or remains asymptomatic, like dysfunctional uterine bleeding(DUB), pelvic discomfort, leukorrhea and hence the reduced rate of getting diagnosed in usual population. A method usually utilized for diagnosis remains the histological examination of the endometrial biopsy (EB), that is thought to be the golden standard diagnostic method by most practitioners currently. But still it is not certain if CE therapy increases live birth rates in the future assisted reproductive treatment(ART) cycles [8,9]. The marked difference observed has been thought to be secondary to the varying methodologies utilized for quantification of plasma cells. CD138+ plasma cells from differing number of roughly decided high power fields (HPF) or the whole biopsied specimen might be chosen by the researchers [8]. Till date there is no acceptable diagnostic criteria by which one can choose what exactly is the criteria with 7 of them being present, of which most have been chosen randomly from the publications on the prevalence of CE.
Method
We utilized a PubMed search engine to find the association of chronic endometritis (CE) with recurrent implantation failure (RIF) with the purpose of best diagnostic methodology and the way it might get treated with the outcome being successful implantation and natural conceptions following its treatment. Thus, the MeSH terms like CE, RIF, criteria of diagnosis of CE utilizing plasma cell density in the endometrial stroma, utilization of next generation sequenting 16S ribosomal RNA subunit, hysteroscopic diagnosis, plasma cell density at 95% level of the fertile women
Results and Discussion
We got a total of 595 articles out of which we selected 53 articles for this minireview. No meta-analysis was done.
Newer Methods of Diagnosis
Hence Liu et al. [10] Established a novel way that includes counting all CD138+ cells in the whole section, then try to get a quantification of the area of tissue section scrutinized, and then give the plasma cell density(PCD) in the form of cells per unit area. For evaluation of the CD138+ cell counts, and the area sectioned a computer software that had been well accepted for the evaluation of scientific image (Image J; US, National Institute of Health] [11]. The correlation coefficients for the intra observer along with interobserver of PCD with this novel way were much> in contrast to the CD138+ cell counts every ten HPF in the usually utilized approach. Importantly plasma cells were there even in women having validated fertility. In view of this a refined threshold (PCD more than 5.15 cells/10mm2 of endometrium, that represented 95th percentile of the reference ranges from a fertile women population [10]. Thus, very strict criteria were used in definition of CE in the current study was utilized. Earlier utilizing microbial culture, the following organisms like Enterococcus faecalis, Enterobacteriaceae, streptococcus spp, staphylococcus spp, Gardnerella vaginalis, mycoplasma spp, ureaplasma urealyticum, Neisseria gonorrhaeae, Chlamydia trachomatis had been isolated from the endometrium of CE women [12]. On the basis of species -particular quantitative polymerase chain reaction (qPCR) assay Moreno et al. [13], formed a molecular diagnosis approach that would challenge 9 bacterial species which had been picked up. From the results received through 113 along with 65 women who had gone through qPCR assays in addition to the 3 typical tests, respectively they showed that these 9 qPCR assays had 77% accuracy for the classification of 13 women having similar diagnostic outcomes of CE based on the usual histology, hysteroscopy, and microbial culture tests. Thus Liu et al. [14] Used endometrial biopsy along with uterine lavage samples in this repeat study from 2018 in 2019, exactly 7 days following the LH surge, at a time when one expects the embryonic implantation, using PCD tested on the basis of Syndecan -1(CD 138)-positive cells in the full biopsy section along with culture independent sequencing of the 16S ribosomal RNA gene done utilizing all endometrial lavage samples(both CE and non CE). With the strict diagnostic criterion, only 12 women (9%)had a diagnosis of CE, in contrast to the much>amounts earlier reported in similar women. Advantage of culture -independent sequencing was that a good performance was obtained from their negative controls with the problem of surrounding contamination overcome.
The median relative excess of Lactobacillus was 1.89% and 80.7% in the CE and nonCE microbiotas respectively. Lactobacillus Crispatus had low abundance in the CE microbial organisms (fold change, range 2.10-2.30). 18 non-Lactobacillus taxa that had Dialister, Bifidobacterium. Preveotella, Garnerella and Anarococcus were higher in the CE microbes(fold change2, 10-18.9).Of which Anaerococcus and Gardenerella negatively associated with the relatively huge amounts of Lactobacillus. Thus, statistically significant higher levels of 18 bacterial taxa in the endometrial cavity was correlated in CE, which one doesn’t consider to be of any surprise as similar report of >non-Lactobacillus species has been documented by man y workers. As concluded by the investigators themselves, next step needed is for evaluation of this subject; that is conduct an intervention trial that is associated with clinical outcomes. Though with the strict diagnostic criteria of CE utilized in this study might have given a population that implicated a more clinically effective diagnosis, more data is required regarding the therapy of CE that was diagnosed using these criteria, in association with the final clinical results .Hence further studies proposed are randomization of patients with CE that are diagnosed using these criteria to either treatment placebo group, and subsequent follow up cure test for validating cure and ensuring resolution. It is essential for checking cure as earlier certain studies that had checked for treatment had not included this in their design, that has made their outcomes getting queried. Further it will be important to blind the ones performing this assessment of therapy to get more proof for a successful therapy of CE. Though there is proof that 90% therapy occurs utilizing a single step therapy with an antibiotic regimen [8], it might be essential to demonstrate cure on application of these strict criteria for diagnosing CE. Hence clinical effectiveness can be only certified with the ultimate clinical outcomes of those getting treatment vs comparison with untreated group. The randomization is the key since various reported experiments, demonstrated that therapy of CE might improve outcomes, randomized studies did not reveal same outcome [15].
The human Microbiome Project demonstrated that 38% of body weight was secondary to bacterial cells, which are at the same numbers as the human somatic cells. These bacterial communities, normally present in the body add to health, once their imbalance occurs it causes different diseases [16]. In the premenopausal women Lactobacillus species are the predominant species in the vaginal cavity [17]. The functions of these Lactobacillus species in vagina is to maintain along with achieve homeostatic levels of the microbial organisms by decreasing the pH via forming lactic acid .Simultaneously the human uterine cavity has been thought to be germ free for long. But recent work has illustrated that microbiota are there in the uterine cavity, mainly being Lactobacillus -dominant [18,19]. Further it was shown that this Lactobacillus -dominant (≥90%) microbes in the endometrial fluid (EF) promoted embryo implantation in the next IVF-ET treatment in infertile women. Conversely, non-Lactobacillus -dominant microbes have a correlation with negative reproductive result that includes implantation failure and miscarriage [20], that promotes the thought omit that endometrial microbial composition is crucial to obtain a good embryo implantation.
RIF occurs in 15-20% of infertile couples going through IVFET treatment [21], Causes of RIF lie in abnormal embryonic factors (e.g. chromosomal abnormalities, mitochondrial DNA quantity, and oxidative stress) [22-24], abnormal endometrial receptivity(like in hydrosalpinx, endometrial polyps, distorted uterine cavity and CE [25-28] along with systemic factors (like thrombophilic and immunological factors)[29,30]. In spite of increasing proof that Lactobacillus species are necessary for the proper vaginal and uterine cavity environments the association between vaginal secretions(VS) microbes and the EF component within same infertile persons are not clear. Thus, Kitaya et al. [2] Utilizing next generation sequencing, tried to find the microbes in the EF and VS in subjects of RIF. 28 subjects of RIF and 18 infertile women undergoing 1st IVF-ET attempt (as control group) were included in this study. On days 6-8 in the luteal phase of the natural, oocyte pick up or hormone replacement cycle, the paired EF and VS samples were taken in different containers. Genomic DNA that was extracted was pyro sequenced for the V4 area of 16S ribosomal RNA with the help of a next generation sequencer. Greater α-diversity with wider bacterial species were observed in the EF microbes as compared to the VS microbes both in the RIF and control groups. The evaluation of the UniFrac distance matrices between EF and VS also displayed significantly separate clustering. In addition, the EF microbes, but not the VS microbes, exhibited significant differences in community composition between the RIF group and the control group. Burkholderia species were not found in the EF microbes of any samples in controls but were found in one fourth of the RIF group. This is the 1st study that has checked the microbes in paired EF and VS samples in infertile women with RIF [31].
Cincinelli E et al. [21] Tried to form a consensus on the diagnostic criteria of CE at hysteroscopy (HSC), and for examination of these criteria determined in a randomized -controller observer study. They conducted a systematic review of studies that had analyzed the diagnostic perfection of HSC in CE diagnosis. Delphi agreement on hysteroscopic diagnostic criteria for CE and randomized -controlled observer study for checking that the diagnostic criteria could be replicated. Experts from various countries participated for developing the Delphi consensus or agreement. Doctors from various countries participated in the observer study. Following development of an agreement on the diagnostic criteria, the Delphi poll made a questionnaire that consisted of 100 hysteroscopic [pictures (50 from women having CE [domain 1]and 50 from without [domain 2], with a single question /picture(Answer A: suggestive of CE; Answer B:not suggestive of CE). Total of 200 physicians were sent invitations to participate in the observer study. Prior to completing the questionnaire, physicians were randomized to get a briefing of the diagnostic criteria (group A) or no such information (group B). The primary outcome consisted of the questionnaire, scores for the 2 groups of observers. The secondary outcome checked the interobserver approval of CE in every group. 126 physicians finished the questionnaire (62 in group A and 64 in group B). Observers from group A achieved > total scores in contrast to group B (P<0.001), but not in domain 2 (P=0.975), Marked agreement was observed among observers in group A(intraclass correlation coefficient (ICC0.78), while a fair agreement was observed among observers in group B(ICC0.40). Thus, concluding that this randomized -controller observer study observed a positive impact of their criteria on physician’s capability to recognize CE [31].
Other investigations in CE with RIF
Repeated implantation failure (RIF), by definition is the absence of achievement of clinical pregnancy following transfer of 3 or > good quality embryos in women under 35yrs of age, and 4 or > good quality embryos in women ≥35yrs during fresh/frozen embryo transfer (ET) cycles [32]. For proper implantation along with pregnancy continuation a fine balance in pro along with anti-inflammatory immune responses occurring at the maternal immune surface are needed [33]. A complicated network of action of endometrial cytokines along with chemokines bring about the alterations in endometrial leukocyte populations, that have a big part in the vascular remodeling along with angiogenesis [34]. But CE causes changes in endometrial cytokines along with chemokines formation, and result in endometrial dysfunction [35]. Additionally, these alterations are correlated with anomalous patterns of lymphocyte subsets and changes in the liberation of paracrine substances. Thus, CE usually correlates with reduced endometrial receptivity for invading embryos and thus recurrent pregnancy losses [36]. In CE women, endometrial immune responses are usually moved towards pro inflammatory profiles and hence are not favorable with respect to the invading embryos [37]. Earlier, reduction in expression of TGF-β and interleukin (IL)-10 that is chiefly liberated by T -helper (Th)-2, Regulatory cells(Tregs) or alternatively stimulated macrophage(M2), and enhanced expression of IL-17 by Th 17 cells have been implicated in taking part in maternal immune rejection of the fetus [38]. Thus, a probable attack on the fetus by maternal immune responses can be avoided by decreasing Th 1 or immune activation [39]. Escalating local as well as systemic Th -2 immunity along with tolerogenic Tregs may be a probable method for reducing the pro inflammatory immune responses.
The cellular homeostatic way that has been preserved markedly, namely autophagy, breakdown large protein aggregations, eliminates damaged or exogenous organelles, gets recycling of nutrients and aids in cell survival at the time of stress [40,41]. In the differentiation and function of T Lymphocytes autophagy acts in a crucial way. Autophagy remains activated in Tregs and promotes their lineage stability along with survival fitness. Genetic /pharmacological changes in autophagy interfere with cell survival rate /its metabolism and thus affect tissue homeostasis [42]. Reduced expression of autophagy correlates with reduced embryonic formation along with its implantation [43]. Microtubule -associated protein 1A /1B -light chain 3(LC3), is a soluble protein which is present in most mammalian tissues. 2 forms of LC3 are present i) a cytosolic form (LC3-1) along with a lipid phosphatidyl ethanolamine-conjugated form (LC3-II) which is embedded in both inner and outer membranes of the growing autophagosomes. This LC3-II, that represent the phosphatidyl ethanolamine- conjugated form of LC3-1, that is formed following activation of LC3 is being used at present as a particular marker for autophagy in view of its action in the production of autophagosomes [44]. A key role of mTOR, that represents the master controller of cellular metabolism, was documented in the control of cellular autophagy [45]. In placenta, mTOR signaling, had a positive correlation with birth weight of the baby [46]. Thus Wang et al. [39] conducted a case control study in a big reproductive medicine Centre from Feb 2015-july 16.75 patients with RIF with CE who displayed a ‘’strawberry aspect’’ (Figures 1&2) and 75 women with male factor infertility got included for this study. Endometrial expressions of IL-17, 1L-10, TGF-β and autophagy associated molecules, including LC3-II and mTOR were examined by qRT-PCR Western blot, immunofluorescence and immunohistochemistry assays. Expression of IL-17 was significantly > in patients with CE in contrast to women having male factor infertility, and the expressions of 1L-10 and TGF-β were significantly <.Further the expression of autophagy (Figure 3) (LC3-II) was enhanced, while that mTORC1 was reduced. Thus, showing that CE correlates with cytokine milieu moved towards Th17 over Treg immunity in endometrium via disturbed autophagy by reduced mTORC1[47].
Figure 1: Courtesy ref no-47 Hysteroscopic finding of chronic endometritis (left) and normal endometrium (right). The focal hyperemic area allowed chronic endometritis to be diagnosed in a woman with a history of repeated implantation failure.
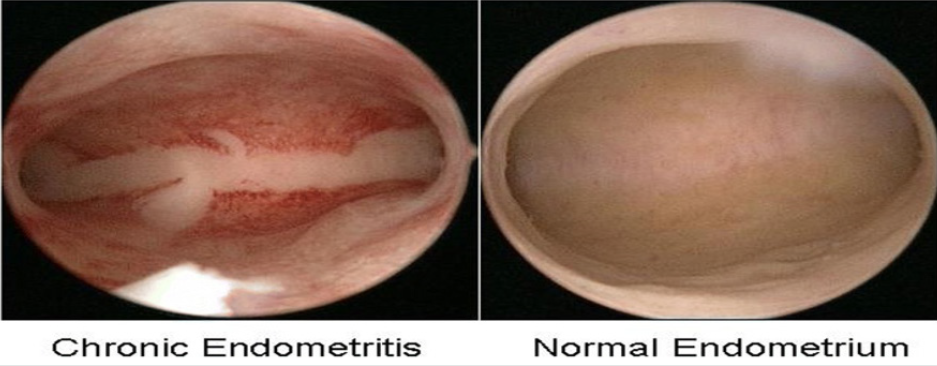
Figure 2: Courtesy ref no-47-Histological analysis of chronic endometritis (left) and normal endometrium (right). The arrow heads allowed chronic endometritis to be diagnosed in a woman with a history of repeated implantation failure.
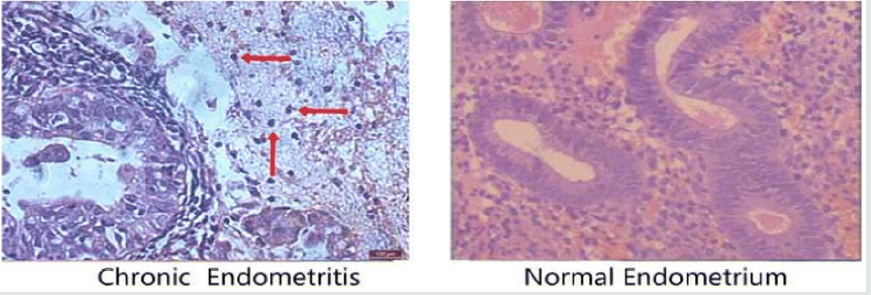
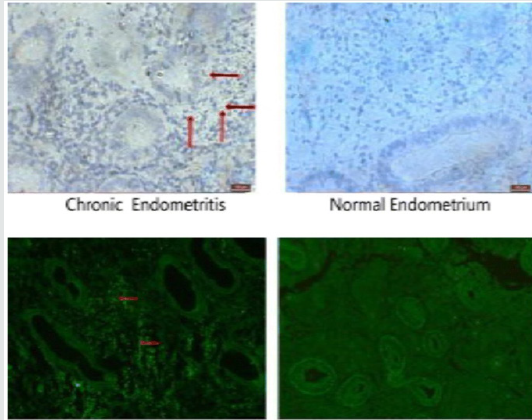
Figure 3: Courtesy ref no-47 Localization of autophagy in CE and normal endometrium. Localization of autophagy marker
(LC3II) by immunohistochemical staining
a. and immunofluorescence
b. (n = 15 for each group). Signs of autophagy are detectable in endometrial stromal samples but not in endometrial glands
Methods of Treatment
A method usually utilized for diagnosis remains the histological examination of the endometrial biopsy, that is thought to be the golden standard diagnostic method by most practitioners currently [48]. But as inflammatory cells are commonly present in endometrium, to diagnose CE becomes difficult. Utilization of hysteroscopy might help further by showing micro polyps, stromal oedema and focal or diffuse hyperemia [12]. Correlation of CE with eosinophils in the endometrium and utilizing antibody of CD138 for PC as discussed earlier to have promise to diagnose with > efficacy. Thus, established criteria for this diagnosis are the need of the hour for attaining proper diagnosis and thus achieve successful therapy in time. Therapy of CE depends on using different antibiotics, based on the type of organism found. These antibiotics are given orally, and later endometrium is checked again after therapy. One aspect to be highlighted is that besides the separate antibiotics used significant differences in dose of every antibiotics used along with variations in the scheme suggested by each practice is there.
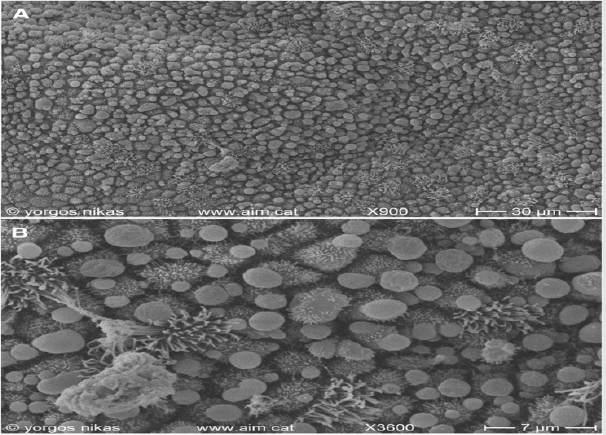
Figure 4: Courtesy [51]
a. SEM micrograph of endometrial surface from a patient with CE. Note the presence of thick mucus layer (right) containing
debris and red blood cells.
b. Detail of (Figure4a). Mucus and abundant bacteria are sticking on the microvilli and the cilia of the endometrial.
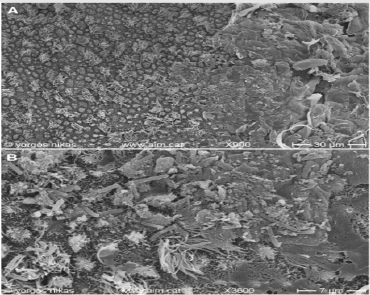
Figure 5: Courtesy [51]
a. Endometrial surface after oral treatment for CE. Note the presence of filamentous mucus (lower) and aggregations of red
blood cells (upper middle).
b. Detail of (Figure 5a). Fibrinous material with red blood cells (middle) -possibly emerging through diapedesis- and
isolated bacteria (middle and lower right) are seen on the epithelium.
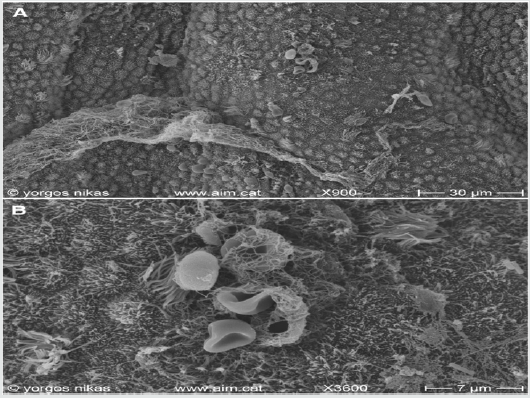
Figure 6: Courtesy [51]
a. Endometrial surface after intrauterine treatment for CE. The surface appears clean with abundant pinopodes.
b. Detail of (Figure 6a). Note the presence of fully developed pinopodes and of a small mucus aggregate (lower left).
After antibiotic usage one expects the endometrial receptivity to improve. But no significant association of any antibiotic usage with positive IVF outcomes have been documented in literature ending in a final agreement that oral antibiotic usage does not improve in vitro fertilization(IVF) outcome [49]. Immune responses that are found in CE do not get converted to systemic inflammation [50]. Hence the query by Staminodes and their group was then why use a systemic antibiotic protocol. Thus, in their case series of 3 subjects who documented multiple earlier failed IVF attempts associated with CE that was not properly managed earlier were presented. After first examination in their clinic and confirmation of CE observations, an oral antibiotic regimen was given on the basis of the infectious agent found. On rechecking little improvement in the microbiological endometrium environment found with inflammation still persistent (Figures 4-6). Thus, intrauterine antibiotic infusion was offered as another way. All their patients became pregnant in a small time period after intrauterine therapy with one patient documenting a live birth of 2 children while 2 patients still having ongoing pregnancy. Thus, they concluded that their case series suggests how this method might help in further infertility treatment related with CE. This line of therapy utilizing oral antibiotics regimens emphasis on evaluating newer methods and warrants larger experiment on the role of them clinically. The method utilized here of intrauterine infusion helped in natural conceptions in patients coming with earlier RIF with multiple failed IVF attempts. Thus, this gives an alternative way to the reproductive endocrinologist for treating RIF needing more probing [51].
Further Cincinelli E et al. [21] tried to evaluate the association between CE and unexplained infertility. They conducted a retrospective study on successive subjects sent to their hysteroscopy department, in view of unexplained infertility. Endometrial sampling, along with histological test and cultures were done in all subjects. In case of CE diagnosis, antibiotics were administered, and that CE had subsided was then evaluated by histological checking. Their idea was to find the prevalence along with antibiotics treatment resulting in spontaneous conception in the year after hysteroscopy. Total 95 women participated. Pooled prevalence of CE was 56.8%. Resolution of CE following antibiotics treatment occurred in 82.3% patients, while persistence of disease was observed in 17.6% patients. Those who had CE cured demonstrated >pregnancy rates along with live birth rates, in contrast to those having persistence of disease and women who did not present with CE diagnosis(pregnancy rates=76.3% vs 20% vs 9.5%, p<0.0001; live birth rates=65.8% vs 6.6% vs 4.8%p<0.0001).Thus concluding that CE has high prevalence in unexplained infertility. Diagnosis and therapy of CE increases the spontaneous pregnancy rates along with live birth rates in these patients [52].
Zhang et al. [47] Carried out a prospective study with the aim to check if the prevalence of CE in RIF patients and to find if intrauterine injection of combined antibiotic with dexamethasone improved clinical pregnancy results .They utilized a combination of hysteroscopy along with histology to diagnose CE in subjects with RIF, and those diagnosed with CE got intrauterine delivery of the combined intrauterine administration of antibiotic and dexamethasone. IF prevalence in CE patients were noted, and the effect of treatment was checked, in the 1st IVF-ET cycle. Of 298 subjects with RIF, 109 subjects (36.58%) were diagnosed as CE. intrauterine delivery of the combined drugs caused a resolution of CE in 77.98% of subjects. Following therapy, 85patients with no signs of CE (Group 2) had a significantly>implantation rate (31.33%) and clinical pregnancy rates (51.76%) in contrast to both 126 cases without CE diagnosis (Group1;16.30% and 30.15% respectively) and 24 cases with persistence of CE (Group3;14.89%and 25.00% respectively .The live birth rates in the 1st IVF-ET cycle after therapy in Group 2 was significantly > as compared to both Group1 and 3 (all p<0.05). Thus, concluding that CE has high prevalence in RIF patients. This was the 1st report of combined intrauterine administration of antibiotic and dexamethasone for therapy of CE as a possible method of treating CE, that could obtain successful pregnancy results [53].
Conclusion
Thus, we have discussed the best methods of diagnosis of CE in cases of RIF regarding diagnosis utilizing either utilizing Hysteroscopic Delphi criteria or the ones suggested by Liu et al. [53] Utilizing plasma cell density within endometrial stroma taking 95% of organisms present in the fertile group. Presence of Lactobacilli in both uterine cavity and vaginal secretions reflects the best microbial milieu for best implantation rates. Few observers have found intrauterine instillation of antibiotics/antibiotics with dexamethasone within uterine cavity gives good spontaneous pregnancy outcomes although this needs further to be tried in greater number of RIF patients before it can be used as a standard method of treatment in cases of RIF with CE in randomized protocols.
References
- Bouet PE, El Hachem H, Monceaue E, Gariepy G, Kadoch IJ, et al. (2016) Chronic Endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. FertilSteril 105(1): 106-110.
- Kitaya K (2011) Prevalence of Chronic Endometritis in recurrent miscarriages. FertilSteril 95(3): 1156-1158.
- Johnson Mac Ananny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM et al. (2010) Chronic Endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. FertilSteril 93(2): 437-441.
- Kitaya K, Matsubayashi H, Yamaguchi K, Nishiyama R, Takaya Y, et al. (2016) Chronic Endometritis: Potential cause of infertility and obstetric and neonatal complications. Am J Reprod Immunol 75(1): 13-22.
- Takebayashi A, Kimura F, Kishi Y, Ishida M, Takahashi A, et al. (2014) The association between endometriosis and Chronic Endometritis. PLoSOne 9(2): e88354.
- McQueen DB, Perfetto CO, Hazard FK, Lathi RB (2015) Pregnancy outcomes in women with Chronic Endometritis and recurrent pregnancy loss. FertilSteril 104(4): 927-931.
- Greenwood SM, Moran JJ (1981) Chronic Endometritis: Morphologic and clinical observations. Obstet Gynaecol 58(2): 176-184.
- Vitagliano A, Saccardi C, Litta PS, Noventa M (2017) Chronic Endometritis: Really so relevant in Repeated IVF failure? Am J Reprod Immunol 78(6): e12758.
- Kasius JC, Fatemi HM, Bourgain C, Sie-Go DM, Eijkemans RJ, et al. (2011) The impact of Chronic Endometritis on Reproductive outcome. FertilSteril 96(6): 1451-1456.
- Liu Y, Chen X, Huang J, Wang CC, Yu MY, et al. (2018) Comparison of the prevalence of Chronic Endometritis as determined by means of different diagnostic methods in women with and without Reproductive failure. Fert Steril 109(5): 832-839.
- Schneider CA, Rachand WS, Elcein KW (2012) NIH Image to Image J:25 years of Image analysis. Nat Methods 9(7): 671-675.
- Cincinelli E, Matteo M, Tinelli R, Pinto V, Marinaccio M, et al. (2014) Chronic Endometritis due to common bacteria is prevalent in women recurrent miscarriages as confirmed by improved pregnancy outcome after antibiotic treatment. Reprod Sci 21(5): 640-647.
- Moreno I, Codoner FM, Vilella F, Valbuena D, Martinez Blanch JF et al. (2016) Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynaecol 215(6): 684-703.
- Liu Y, Yee Ling Ko, Ka WingWong K, Chen X, Wing Ching C, et al. (2019) Endometrial microbiota in infertile women with and without Chronic Endometritis as diagnosed using a MaybinJAquantitative and reference range –based method. FertilSteril 112(4): 707-717.
- Monteno I, Cincinelli E, Garcia Grau I, Gonzalez Monfort M, Bau D, et al. (2018) The diagnosis of Chronic Endometritis in infertile asymptomatic: a comparative study of histology, cultures, hysteroscopy and molecular microbiology. Am J Obstet Gynaecol 218(6): 602.e1-16.
- Franasiak JM (2019) Chronic Endometritis is associated with an alterdmicrobiome,but what about treatment and outcomes? FertilSteril 112(4): 649-650.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser Liggert CM, Knight R, et al. (2007) The Human Microbiome Project. Nature 449(7164): 804-810.
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, et al. (2011) Vaginal microbiome of reproductive age women. Proc Natl Acad Sci USA 108(Suppl1): 4680-4687.
- Franasiak JM, Werner MD, Juneau R, Tao X, Landis J et al. (2016) Endometrial Microbiome at the time of embryo transfer: next generation sequencing of 16S ribosomal subunit. J Assist Reprod Genet 33(1): 129-136.
- Chen C, Song X, Wei W, Zhong H, Dai J, et al. (2017) The microbiota continuum along the female reproductive tract and its relation to uterine related diseases. Nat Commun 8(1): 875.
- Cincinelli E, Vitagliano A, Kumar A, Lasmar RB, Bettochi S, et al. (2019) International Working group for Standardization of Chronic Endometritis Diagnosis. Unified diagnostic criteria for Chronic Endometritis at fluid hysteroscopy: proposal and reliability evaluation through an international randomized –controller observer study. FertilSteril 112(1): 162-73.
- Kort JD, McCoy RC, Demko Z, Lathi RB (2018) Are blastocyst aneuploidy rates different between fertile and infertile populations. J Assist Reprod Genet 35(3):403-408.
- Ruder EH, Hartman TJ, Blumberg J, Goldman MB (2008) Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update 14(4): 345-357.
- Wells D (2017) Mitochondrial DNA quantity as a biomarker for blastocyst implantation potential. FertilSteril 108(5): 742-747.
- Xu B, Zhang Q, Zhao J, Wang Y, Xu D, et al. (2017) Pregnancy outcome of in vitro fertilization after Esure and laparoscopic management of hydrosalpinx: a systematic review and meta-analysis. FertilSteril 108(1): 84-95.
- Di SpiezioSardo A, Di Carlo S, Minozzi S, Spinelli M, Pistotti V et al. (2016) Efficacy of hysteroscopy in improving reproductive outcomes of infertile couples: a systematic review and meta-analysis. Hum Reprod Update 22(4): 479-496.
- Pritts EA, Parker WH, Olive DI (2009) Fibroids and infertility: an updated systematic review of the evidence. FertilSteril 91(4): 1215-1223.
- Kitaya K, Takeuchi T, Mizuta S, Matsubayashi H, Ishikawa T (2018) Endometritis: new times ,new concepts. FertilSteril 110(3): 344-350.
- Fatemi HM, Popovic Todorovic B (2013) Implantation in assisted reproduction: a look at endometrial receptivity. Reprod Biomed Online 27(5): 530-538.
- Ivanov P, Tsvyatkovska T, Konova E, KomsaPenkova R (2012) Inherited thrombophilia and IVF Failure the impact of coagulation disorders on implantation process. Am J Reprod Immunol 68(3): 189-198.
- Kitaya K, Nagai Y, Arai W, Sakuraba Y, Ishikawa T et al. (2019) Characterization of Microbiota in Endometrial Fluid and vaginal secretions in Repeated implantation failure. Mediators of Inflammation p.10.
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, et al. (2014) Recurrent implantation failure: Definition and management. Reprod Biomed Online 28(1): 14-38.
- Liu S, Diao L, Huang C, Li Y, Zeng Y, et al. (2017) The role of decidual immune cells on human pregnancy. J Reprod Immunol 124: 44-53.
- Dunk C, Smith S, Hazan A, Whittle W, Jones RL et al. (2008) Promotion of angiogenesis, by human endometrial lymphocytes. Immunol Investig 37(5-6): 583-610.
- Maybin JA, Critchley HO, Jabbour HN (2011) Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol 335(1): 42-51.
- Matteo M, Cincinelli E, Greko P, Massenzio F, Baldini D, et al. (2009) Abnormal patterns of lymphocyte szubpopulations in the endometrium of infertile women with Chronic Endometritis. Am J Reprod Immunol 61(5): 322-329.
- Park HJ, Kim YS, Yoon TK, Lee WS (2016) Chronic Endometritis and infertility. Clin Exp Reprod Med 43(4): 185-192.
- Hori S, Nomura T, Sagakuchi S (2003) Control of regulatory Tcell development by the transcription factor FoxP3. Science 299(5609): 1057-1061.
- Wang WJ, Hao CF, Qu QL, Wang X, Qiu LH, et al. (2010) The downregulation of regulatory Tcell on interleukin producing T helper cells in patients with unexplained early recurrent miscarriages. Hum Reprod 25(10): 2591-2596.
- Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4): 728-741.
- Deretic V, Saitoh T, Akira S (2013) Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13(10): 722-737.
- Wei J, Long L, Yang K, Guy C, Shrestha S, et al. (2016) Autophagy enforces functional integrity of regulatory Tcell by coupling environmental cues and metabolic homeostasis. Nat Immunol 17(3): 277-285.
- Tsukamoto S, Kiuma A, Murakami M, Kishi C, Yamamoto A, et al. (2008) Autophagy is essential for preimplantation development of mouse embryos. Science 321(5885): 117-120.
- Tanda I, Ueno T, Kominami E (2008) LC3 and Autophagy. Methods Mol Biol 445: 77-88.
- Wood SC, Sheeley RJ, Cota D (2008) Regulation of food intake through hypothalamic signalling involving mTOR. Annu Rev Nutr 28: 295-331.
- Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, et al. (2013) Activation of placental mTOR signalling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab 98(1): 105-113.
- Wang WJ, Zhang H, Chen ZQ, Zhang W, Liu XM, et al. (2019) Endometrial TGF-β, IL-10, IL-17 and Autophagy are dysregulated in women with recurrent implantation failure with Chronic Endometritis. Reprod Biol and Endocrinol 17(1): 2.
- Kasius JC, Broekmans F, Sie Go DM, Bourgain C, Eijkemans RJ, et al. (2011) The reality of the histological diagnosis of Endometritis in asymptomatic IVF cases:a multicenter observer study. Hum Reprod 27(1): 153-158.
- Lamonica R, Hartnett J, Engmann L, Sanders M, Maier D (2006) Immunohistochemistry confirms the presence of Chronic Endometritis in patients with recurrent implantation failure. FertilSteril 86(3): S280.
- Kushnir VA, Solouki S, Sarig Meth T, Vega MG, Albertini DF, et al. (2016) Systemic inflammation and autoimmunity in women with Chronic Endometritis. Am J Reprod Immunol 75(6): 672-677.
- Sfakianoudis K, Simopoulo M, Nikas Y, Rapani A, Nitsos N, et al. (2018) Efficient treatment of Chronic Endometritis through a novel approach of intrauterine antibiotic infusion: a case series. BMC Women’s Health 18(1): 197.
- Cincinelli E, Matteo M, Trojano G, Mitola PC, Tinelli R, et al. (2018) Chronic Endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am J Reprod Immunol 79(1).
- Zhang Y, Xu H, Liu Y, Zheng S, Zhao W, et al. (2019) Confirmation of Chronic Endometritis in Repeated implantation failure and success outcome after intrauterine delivery of the combined administration of antibiotic and dexamethasone. Am J Reprod Immunol 82(5): e13177.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...