Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1644
Case ReportOpen Access
Diagnosis and Management of Vaginal Endometriosis Involvement in Postmenopausal Woman. A Case report Volume 2 - Issue 3
Baquedano Mainar L*, Herrero Serrano R, Espiau Romera A, Gabasa Gorgas L, Pallarés Arnal V, Benito Vielba M, Ortega Marcilla S and Lamarca Ballesteros M
- Department of Gynecology, Miguel Servet University Hospital, Zaragoza, Spain
Received: March 01, 2019; Published: March 07, 2019
Corresponding author: Laura Baquedano Mainar, Gynecology Department, Paseo Isabel La Catholica 1-3, 50009, Miguel Servet University Hospital, Zaragoza, Spain
DOI: 10.32474/OAJRSD.2019.02.000138
Abstract
Endometriosis is a pathology which affects up to 10% of the female population in reproductive age characterized by the presence of actively functional endometrial ectopic tissue that suffers cyclical changes that are induced by the ovarian hormones, which produces a chronic inflammatory reaction. This disease occurs during the reproductive years and is rarely diagnosed after menopause.
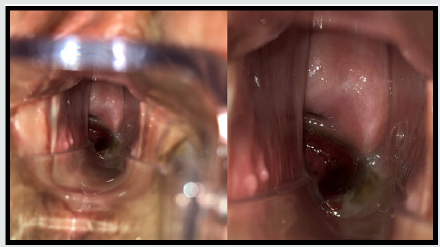
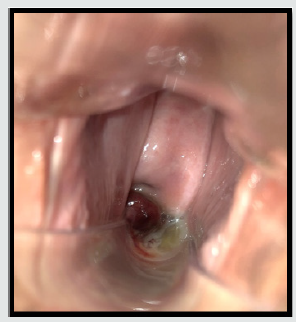
Case Report: A 51 years old woman, who had had a unilateral laparoscopic adnexectomy due to an endometrioma one year before, was admitted because of postmenopausal vaginal bleeding. The medical examination only showed two polypoid formations of 1 and 3cm located on posterior vaginal fornix which presented with smooth cystic surface. A histological analysis was carried out revealing an endometriotic nature. Despite the bleeding, the patient was asymptomatic, consequently a close monitoring was adopted.
Discussion: Typically, endometriosis resolves after natural or iatrogenic menopause due to declining estrogen levels. Nonetheless, case reports over the years have highlighted the incidence of recurrent postmenopausal endometriosis. Occurrence or progression of postmenopausal endometriosis lesions could be related to extra-ovarian production of estrogen by endometriosis lesions and adipose tissue, which becomes the major estrogen-producing tissue after menopause. Hormone therapy (HT) may reactivate endometriosis and stimulate malignant transformation in women with a history of endometriosis.
The risk of malignant transformation of premenopausal endometriosis is around 1%. Furthermore, patients with endometriosis have an increased risk of ovarian cancer and other malignancies. These transformations appear to be further elevated in patients who take HT, although this issue is not fully elucidated. The evidence is currently insufficient to support a conclusion about the optimal HT for women with endometriosis. Given the uncertain risks of initiating it, it is laborious to determine the best management.
Conclusión: Vaginal involvement is an atypical endometriosis location, especially in menopausal women, thus close surveillance is necessary.
Keywords:Endometriosis; Menopause; Vaginal Diseases; Vaginal Bleeding
Introduction
Endometriosis is a pathology which usually occurs during the reproductive period and is rarely diagnosed after menopause [1]. The prevalence of postmenopausal endometriosis is around 2-5% and it is not known yet whether it is a progression of a previously existent endometriosis or it develops de novo [2]. It is thought that the pathogenic mechanism of postmenopausal endometriosis may involve “estrogen threshold theory”. According to this theory a reactivation of endometriosis islets is produced, even in the lower estrogenic menopausal state. Nevertheless, de novo endometriosis lesions after menopause can also be produced [3]. The main clinical manifestations consist of endometrial implants, being the pelvic organs the most frequent locations. However, different locations of endometriosis are described, as cutaneous, intestinal or ureteral foci.
The management of endometriosis after menopause is still controversial but, in most cases, the first line of treatment includes surgery [4]. Recurrences are common and second-line drug treatment could be necessary [5]. Although endometriosis is a benign disease, postmenopausal endometriosis brings an increased risk for malignancy up to 1% of cases. Several cases of neoplasia on postmenopausal lesions have been reported, being ovarian cysts the most frequent way in which a malignant lesion can appear. This finding should encourage clinicians to have a close surveillance of postmenopausal women who suffer from endometriosis [6]. We introduce an uncommon location of postmenopausal endometriosis and its management.
Case and Methods
A case of a 51-years-old woman who consulted due to postmenopausal bleeding is conducted. Highlighted as personal background, the patient was Gravida 1 Para 1 (C-section), postmenopausal for 2 years and had rheumatoid arthritis and dyslipidemia following treatment with methotrexate and statins. The year before unilateral right laparoscopic adnexectomy was performed due to a five cm expansive cyst diagnosed by magnetic resonance (MR). Laparoscopic findings showed a normal uterus and left ovary while the right ovary presented a multicystic solid formation described as “a bunch of grapes”. The final pathological analysis informed of an endometrioid fibrous lesion. Rest of exploration was out of any endometriosis foci.
At present time, the patient came at emergency unit with postmenopausal vaginal bleeding. The medical examination only showed two polypoid formations of 1 and 3cm located on posterior vaginal fornix which presented a smooth cystic surface (Figures 1 & 2). The rest of the vaginal exploration was anodyne. Transvaginal ultrasound showed a 3.5cm intramural-sub serosal leiomyoma located in the anterior wall of the uterus and a 6.2mm endometrial line. No other endometrial implants were found by the ultrasound examination. The pathological analysis of the vaginal injury was carried out revealing its endometrial nature with CD 10 positive reaction in peri glandular stromal cells and either estrogen or progesterone receptors in stromal cells and glands. A routinely endometrial sample was taken which revealed no proliferative activity and atrophic endometrial mucosae. Despite the bleeding, the patient stayed asymptomatic. Consequently, a close monitoring was adopted to be performed every three months.
Discussion
Typically regarded as a premenopausal disease, endometriosis resolves after natural or iatrogenic menopause due to declining estrogen levels. Nonetheless, case reports over the years have highlighted the incidence of recurrent postmenopausal endometriosis. According to these studies, the prevalence of postmenopausal endometriosis is 2-5% [7]. Asymptomatic endometriosis lesions can be discovered incidentally during pelvic imaging or surgical interventions performed on postmenopausal women for other conditions. Many different locations of postmenopausal endometriosis were described [8-10]. Data on the physio pathological mechanisms implicated in postmenopausal endometriosis are limited. Postmenopausal endometriosis is even more complex, because it is not known whether it is a continuation of a previous disease or it develops de novo. After menopause, endometriosis lesions can be stimulated by estrogen from extraovarian sources including adipose tissue, the adrenal glands, or an exogenous source (e.g. hormone menopause therapy, MHT) [11]. Another mechanism has been suggested is the estrogen production by the endometriosis lesions. According to Bulun and colleagues, aromatase is expressed in endometriosis implants and in the ectopic endometrium of women with endometriosis; autocrine and paracrine effects result in local production of estrogen [12,13].
The risk of malignant transformation of premenopausal endometriosis is around 1% occurring most commonly in ovarian lesions [14]. Furthermore, patients with endometriosis have an increased risk of ovarian cancer and other malignancies. The risk of malignant transformation increases after menopause and after introduction of MHT, especially in case of unopposed estrogen substitution [15]. Since there have been contradictory reports on postmenopausal HT used in women with previously known endometriosis, in 2010, European Menopause and Andropause Society reported a position statement regarding to managing menopause in women with previous history of endometriosis [16]. As pointed out in the European Menopause and Andropause Society’s statement, today we accept that hormonal therapy may reactivate residual lesions, and the risk of malignant transformation of endometriosis had to be considered in postmenopausal women. The most common malignancies associated with endometriosis were endometrioid and clear-cell ovarian cancers [17]. Several cases of neoplasia in postmenopausal endometriosis lesions have been reported in the literature and are summarized in a review article by Soliman, et al. [18].
First line treatment for symptomatic endometriosis in postmenopausal patients should be surgical, mainly due to the risk of malignancy. However, surgery in such patients may carry some risks. Firstly, such cases are older when compared with cases at reproductive ages, thus may have comorbidities. Secondly, these patients may have previous surgeries, accordingly, may have higher operative risks. Nevertheless, recurrences are common after surgical treatment and second-line drug treatment may be necessary. Medical treatment has been studied in women with endometriosis in post menopause. Due to hormonal peripheral conversion, aromatase inhibitors may propose a new alternative for postmenopausal patients with endometriosis [19,20].
Conclusion
Vaginal endometriosis in postmenopausal women is an extremely rare condition. Malignant transformation seems to be increased. The evidence is currently insufficient to support conclusions about the best management in these patients. Multicenter studies should be performed about endometriosis and menopause.
References
- Tan DA, Almaria MJG (2018) Postmenopausal endometriosis: drawing a clearer clinical picture. Climacteric 21(3): 249-255.
- Inceboz U (2015) Endometriosis after Menopause. Women’s Heal 11(5): 711-715.
- Streuli I, Gaitzsch H, Wenger JM, Petignat P (2017) Endometriosis after menopause: physiopathology and management of an uncommon condition. Climacteric 20(2): 138-143.
- Gemmell LC, Webster KE, Kirtley S, Vincent K, Zondervan KT, et al. (2017) The management of menopause in women with a history of endometriosis: a systematic review. Hum Reprod Update 23(4): 481- 500.
- Shah D (2012) Postmenopausal endometriosis-a new dimension. Climacteric 15(5): 507-508.
- Yoldemir T (2018) Quality of life for women with endometriosis: premenopausal and postmenopausal perspectives. Climacteric 21(5): 411-412.
- Haas D, Chvatal R, Reichert B, Renner S, Shebl O, et al. (2012) Endometriosis: a premenopausal disease? Age pattern in 42,079 patients with endometriosis. Arch. Gynecol. Obstet 286(3): 667-670.
- Popoutchi P, dos Reis Lemos CR, Silva JC, Nogueira AA, Feres O, et al. (2008) Postmenopausal intestinal obstructive endometriosis: case report and review of the literature. Sao Paulo Med. J 126(3): 190-193.
- Beuke M, Dahlem R, Fisch M (2006) Distal stenosis of the ureter due to extrinsic endometriosis in a postmenopausal woman. Aktuelle Urol 37(2): 143-144.
- Pugliese JM, Peterson AC, Philbrick JH Jr, Allen RC Jr (2006) Ureteral endometriosis in patients after total abdominal hysterectomy: presentation and diagnosis: a case series. Urology 67(3): 622.e13-15.
- Bendon CL, Becker CM (2012) Potential mechanisms of postmenopausal endometriosis. Maturitas 72(3): 214-219.
- Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, et al. (2002) Estrogen production and metabolism in endometriosis. Ann N Y Acad Sci 955: 396-406.
- Bulun SE (2009) Endometriosis. N Engl J Med 360: 268-279.
- Pollacco J, Sacco K, Portelli M, Schembri-Wismayer P, Calleja-Agius J (2012) Molecular links between endometriosis and cancer. Gynecol Endocrinol 28(8): 577-581.
- Kobayashi H, Sumimoto K, Kitanaka T, Yamada Y, Sado T, et al. (2008) Ovarian endometrioma-risks factors of ovarian cancer development. Eur J Obstet Gynecol Reprod Biol 138(2): 187-193.
- Moen MH, Rees M, Brincat M, Erel T, Gambacciani M, et al. (2010) European Menopause and Andropause Society. EMAS position statement: managing the menopause in women with a past history of endometriosis. Maturitas 67(1): 94-97.
- Zanetta GM, Webb MJ, Li H, Keeney GL (2000) Hyperestrogenism: a relevant risk factor for the development of cancer from endometriosis. Gynecol Oncol 79(1): 18-22.
- Soliman NF, Hillard TC. (2006) Hormone replacement therapy in women with past history of endometriosis. Climacteric 9(5): 325-335.
- Special report (2015) Endometriosis after menopause. Women’s Health 11(5): 711-715.
- Attar E, Bulun SE (2006) Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil. Steril 85(5): 1307-1318.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...