Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1644
Research ArticleOpen Access
Apoptosis in Human Cumulus Cells and Intracytoplasmic Sperm Injection Outcomes Volume 2 - Issue 5
Eman Anwar Hassan1, Mohamed SM Nasr2 and Behery MA3*
- 1Embryology unit, the International Islamic Institute for Population Studies and Research,Al-Azhar University, Egypt
- 2Department of Histology and Cell biology, Faculty of Medicine, Al-Azhar University, Egypt.
- 3ART unit, the International Islamic Institute for Population Studies and Research, Al-Azhar University, Egypt
Received: November 05, 2019; Published: April 07, 2020
Corresponding author:Behery MA, ART unit, the International Islamic Institute for Population Studies and Research, Al-Azhar University, Heliopolis, Cairo, Egypt
DOI: 10.32474/OAJRSD.2020.02.000148
Abstract
Apoptosis as a genetically controlled form of cell death is closely associated with most of the reproductive processes. It has been reported that the incidence of apoptosis in granulosa or Cumulus cells may be used as a predictive factor in the outcome of assisted reproduction cycles. In this study it was revealed that the incidence of apoptosis in cumulus increased with increased female age and that influences fertilization rate, number of MII oocytes in addition to the number of Grade A embryos.
Keywords: Oocyte-Cumulus, Age; Fertilization; ICSI; Outcomet
Abbreviations: CC: Cumulus cells; ICSI: intracytoplasmic sperm injection; cAMP: cyclic adenosine monophosphate; OCC: Oocytecumulus complexes; SOD: superoxide dismutase
Introduction
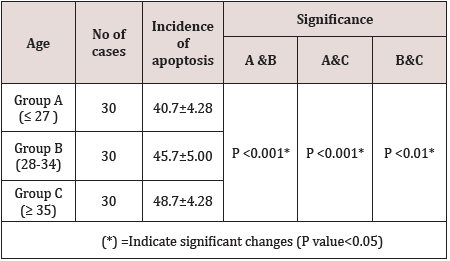
Apoptosis is defined as cell death form that involves variety of signaling pathways and has a major role in the normal function of all tissues. It is associated with reproductive process in view of high incidence of apoptosis in the human cumulus cells (CC) is associated with their alteration and in turn poor development of oocytes and embryos [1,2]. Many studies reported that increased apoptosis in CC is associated with poor reproductive outcome [3]. As the morphological feature of oocytes is correlated with fertilization rate and implantation rate during ICSI cycles. So the apoptosis in CC an affect oocyte maturation, fertilization and embryo development [4]. In the ovary, apoptosis can be initiated in four different cells, i.e., the theca cells, the granulosa cells, the cumulus cells, and the oocyte itself [5]. We can describe the process of apoptosis in four stages, stage one include DNA fragmentation, stage two, decrease in cell volume, stage three, loss of mitochondrial function, stage four, cell membrane alteration and blebbing and the last stage include cell breakage due to increased apoptotic bodies that is phagocytosed by phagocytes [6]. The rational of this study is to evaluate the effects of apoptosis on the oocyte quality and its relation to age and in turn, their effects on fertilization rate and ICSI outcomes (Table 1& Figure 1).
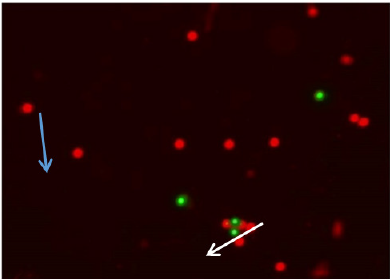
Figure 1: Immuno-fluorescent micrograph shows cumulus cells of group A. Apoptotic cumulus cells show green fluorescence (white arrow); whereas normal cells appear reddish color (blue arrow). (Magnification x 10) stained with Mitochondrial Apoptosis Detection Fluor metric Kit (Bio Vision).
Materials and Methods
This is a prospective clinical trial included 90 couples who were referred for assisted reproduction at the fertility clinic in International Islamic Center for Population Studies and Research, Al-azhar University, between May and December 2016 with the following criteria:
Inclusion Criteria
1-any subjects indicated for ICSI including couples with male
factor infertility.
2-female ages ranged between 23 and 42 years with regular
menstrual cycles.
Exclusion Criteria
Cumulus cells of atretic or empty follicles were excluded from
the study. The 90 female subjects were divided into 3 groups
according to age:
i. Group A: female partner ≤ 27 y (30 cases)
ii. Group B: female partner 28-34 y (30 cases)
iii. Group C: female partner ≥ 35 y (30 cases). The main
outcome measure was the high quality embryos. The secondary
outcome measures were the relation between Incidence
of cumulus cells apoptosis and age, number and quality of
oocytes and fertilization rate.
Assessment of Apoptosis in Cumulus Cells
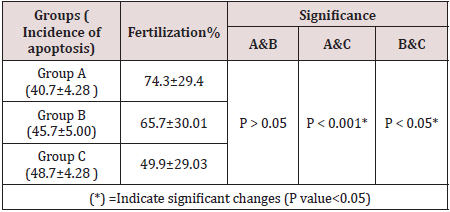
In the retrieved oocytes cumulus cells were stained to detect apoptosis, we used the Mito Capture Mitochondrial Apoptosis Detection Fluor metric Kit (Bio Vision Incorporated, 155 S. Milpitas Boulevard, Milpitas, CA 95035 USA).Disruption of the mitochondrial transmembrane potential is one of the earliest intracellular events that occur following induction of apoptosis. The Mito Capture Apoptosis Detection Kit provides a simple, fluorescent-based method for distinguishing between healthy and apoptotic cells by detecting the changes in the mitochondrial transmembrane potential. The kit utilizes Mito-Capture TM, a cationic dye that fluoresces differently in healthy vs apoptotic cells. In healthy cells, Mito Capture accumulates and aggregates in the mitochondria, giving off a bright red fluorescence. In apoptotic cells, Mito Capture cannot aggregate in the mitochondria due to the altered mitochondrial transmembrane potential, and thus it remains in the cytoplasm in its monomer form, fluorescing green. The fluorescent signals were easily detected by fluorescence microscopy using a band-pass filter (detects FITC and rhodamine) (Table 2 and Figure 2&3).
Figure 2: Immuno-fluorescent micrograph shows cumulus cells of group B. Apoptotic cumulus cells show green fluorescence (white arrows); whereas normal cells appear reddish color (blue arrow). (Magnification x 10) stained with Mitochondrial Apoptosis Detection Fluor metric Kit (Bio Vision).
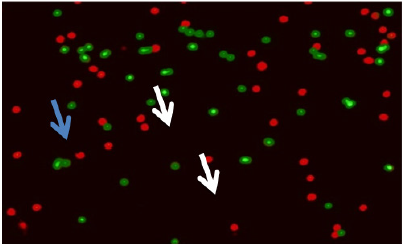
Figure 3: Immuno-fluorescent micrograph shows cumulus cells of group C. Apoptotic cumulus cells show green fluorescence (white arrows); whereas normal cells appear reddish color (blue arrow). (Magnification x 10) stained with Mitochondrial Apoptosis Detection Fluor metric Kit (Bio Vision).
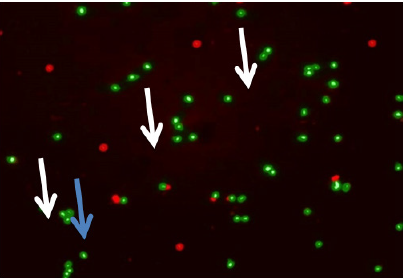
Discussion
The meiotic arrest of oocytes is regulated by cyclic adenosine
monophosphate (cAMP), which is produced by cumulus cells.
There is an intimate relation between a cumulus cell syncytium
in conjunction with the oocyte involving large gap junctions. The
gap junctions open and the large molecules move freely between
the cumulus cells through the zona pellucida into the oocyte
when stimulated with FSH, and before LH peak [7]. Because of
the different physiologic effect of hormones on apoptosis, i.e.
stimulatory (androgens, GnRH, or GnRH analogues) and inhibitory
(E2, FSH, LH and hCG) effects, the results of many studies evaluating
the correlation between the levels of apoptosis in granulosa and
cumulus cells in relation to age and ICSI outcomes are contradictory
[8-11]. Also this difference in study results may be attributed to
the different methods of measuring apoptosis like Comet assay,
flow cytometry, Hoestcht, Apodkit staining, or TUNEL assay that
may have been used. The origin of cumulus cells either from the
pool of collected follicles or from individual follicle may also affect
results [12]. The incidence of apoptosis in CC is increasing with
increased age and its relation to poor oocyte quality was reported
in many studies [4,13]. On contrary to these results ,another
studies reported no relation between the age of the subjects and
apoptotic level in cumulus cells [14-16]. In this study, there was
no concomitant relation observed between the number of oocytes
retrieved and the incidence of cumulus cell apoptosis. If an oocyte
falls to atresia, it might disappear during follicular development.
As a result, cumulus cells are not collected from Oocyte-cumulus
complexes (OCC) of atretic follicles at oocyte retrieval. Thus, the
incidence of apoptosis of cumulus cells is not associated with the
reduction of oocyte number resulting from follicular atresia. The
same result was reported by many studies that revealed that no
correlation observed between the number of oocytes retrieved
(more or less than five) and level of cumulus cell apoptosis [14,15].
With different results, a study by Nakahara, et al. 1997 reported
a relationship between the numbers of oocytes retrieved and the
incidence of ovarian cells apoptosis [17]? They argued that the
incidence of granulosa cell apoptosis was increased as the number
of retrieved oocytes decreases. This decreased oocyte number was
related to the increased number of empty follicles resulting from
the apoptosis in granulosa cells during follicular development.
In our study, there was no concomitant relation observed
between incidence of cumulus cells apoptosis and oocyte
morphology. This finding may be due to the source of collected
cumulus cells as they were derived from a pool of aspirated follicles.
Our results differ from the data of a study by Lee et al 2003 which
suggest that the incidence of cumulus cells apoptosis could predict
the age-related decline in fertility as well as oocyte quality [4] The
greater incidence of cumulus cells apoptosis with reproductive
aging may induce unfavorable environments for follicular oocyte
development, which results in deterioration of oocyte quality,
thus reducing fertilization rates and embryo development. As the
fertilizing ability of oocytes is positively correlated with the cumulus
cell’s DNA status .And this status is affected by increased apoptosis
.So we can explain the inverse correlation between the incidence of
apoptosis and fertilization rate which was demonstrated by many
studies and researches. Therefore the incidence of cumulus cells
apoptosis is associated with decreased fertilization rate. Also our
result confirm the data of related studies by Bosco, et al. 2005 [18].
which demonstrated that apoptosis in human oocytes determines
fertilization failure after ICSI.
The incidence of cumulus cell apoptosis was significantly higher in unfertilized oocytes than in fertilized oocytes as evidenced by many studies. Also a lower incidence of apoptosis in cumulus cells from fertilized oocytes compared with non-fertilized oocytes had been reported by several investigators [4,14]. However, another study by Corn, et al. did not find any correlation between apoptosis in cumulus cells and fertilization rate [13].
In spite of inverse relationship between apoptotic rate in cumulus cells and embryo quality at day 2 of the cleavage stage that has been reported by Nakahara, et al, and Lee, et al. [4,14] our result did not support this finding. Similar results were reported by many studies [4-19] who reported no correlation between apoptosis in cumulus cells and embryo quality at day 3 of embryo cleavage. Factors other than apoptosis may contribute in ICSI failure such as: oxidative stress -induced digestion of DNA also increase with the increased female age and this is accompanied by decrease of CCs anti-oxidant level like superoxide dismutase (SOD) which protect the oocyte from damage caused by reactive oxygen species. Similar findings were reported by Matos, et al.. Also gap junction communication between the oocyte & CCs are required for normal oocyte and follicle
Conclusion
In this study it was revealed that the incidence of apoptosis in cumulus increased with increased female age and that influences fertilization rate, number of MII oocytes in addition to the number of Grade A embryos.
Conflict of interests
The authors reports no conflicts of interest.
Acknowledgmentc
The authors introduce many thanks to all staff in histology and embryology departments at Al Azhar University for their efforts and time until finishing this study.
References
- Bo Zhaia, Huiyu Liua, Xu Lib, Lisheng Daia, Yan Gaoa, et al. (2013) Cell Physiol Biochem 32: 264-278.
- Yeo CX, Gilchrist RB, Lane M (2009) Disruption of bidirectional oocyte–cumulus paracrine signaling during in vitro maturation reduces subsequent mouse oocyte developmental competence. Biol Reprod 80(5): 1072-1080.
- Xu lei Sun, Hao Jiang, Dong xu Han, Yao Fu, Jian bo Liu, et al. (2018) The activated DNA double-strand break repair pathway in cumulus cells from aging patients may be used as a convincing predictor of poor outcomes after in vitro fertilization-embryo transfer treatment. PLoS One 13(9): e0204524.
- Laura Rienzi, Gábor Vajta, Filippo Ubaldi (2011) Predictive value of oocyte morphology in human IVF: A systematic review of the literature. Hum Reprod Update 17(1): 34-45.
- Yuting Fan, Yajie Chang, Lina Wei, Jianhui Chen, Jingjie Li, et al. (2019) Apoptosis of mural granulosa cells is increased in women with diminished ovarian reserve. Journal of Assisted Reproduction and Genetics 36(6): 1225-1235.
- Yingying Zhan, Xin Chen, Cyril Gueydan, Jiahuai Han (2018) Plasma membrane changes during programmed cell deaths. Cell Research 28(1): 9-21.
- Pilar FP, Xuan KN, Tomoki N, Thi TMB, Takuya W, et al. (2019) Removal of cumulus cells around 20 h after the start of in vitro maturation improves the meiotic competence of porcine oocytes via reduction in cAMP and cGMP levels. J Reprod Dev 65(2): 177-182.
- Diego E, Peter H (2014) A review of luteinising hormone and human chorionic gonadotropin when used in assisted reproductive technology. Reprod Biol Endocrinol 12: 95.
- Kaneko T, Saito H, Toya M, Saito T, Nakahara K, et al. (2000) Hyaluronic acid inhibits apoptosis in granulosa cells via CD44. J AsssistReprod Genet 17(3): 162-167.
- Daniele Santi, Livio Casarini1, Carlo Alviggi, Manuela Simoni (2017) Efficacy of Follicle-Stimulating Hormone (FSH) Alone, FSH + Luteinizing Hormone, Human Menopausal Gonadotropin or FSH + Human Chorionic Gonadotropin on Assisted Reproductive Technology Outcomes in the “Personalized” Medicine Era: A Meta-analysis. Front. Endocrinol 8: 114.
- Ruvolo G, Bosco L, Pane A, Morici G, Cittadini E, et al. (2007) Lower apoptosis rate in human cumulus cells after administration of recombinant lutelizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. FertilSteril 87(3): 542-546.
- Marina Diaz Fontdevila, Ricardo Pommer, Rosita Smith (2009) Cumulus cell apoptosis changes with exposure to spermatozoa and pathologies involved in infertility. Fertility Sterility 91(5): 2061-2068.
- MG Da Broi, VSI Giorgi, F Wang, DL Keefe, D Albertini, et al. (2018) Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod Genet. 35(5): 735-751.
- Sheena LP, Regan, Phil G Knight, John LYovich, Yee Leung, (2018) Granulosa Cell Apoptosis in the Ovarian Follicle-A Changing View. Front Endocrinol (Lausanne) 9: 61.
- Bárbara Lourenço, Ana Paula Sousa, Teresa Almeida Santos, João Ramalho Santos (2014) Relation of Cumulus Cell Status with Single Oocyte Maturity, Fertilization Capability and Patient Age. J Reprod Infertil. 15(1): 15-21.
- Abu Hassan D, Koester F, Shoepper B, Schultze-Mosgau A, Asimakopoulos B, et al. (2006) comet assay of cumulus cells and spermatozoa DNA status, and the relationship to oocyte fertilization and embryo quality following ICSI. Repord Biomed online 12: 447-452.
- Bosco L, Ruvolo G, Morici G, Manno M, Cittadini E, et al. (2005) Apoptosis in human unfertilized oocytes after intracytoplasmic sperm injection. Fertil Steril 84(5): 1417-1423.
- Marina Diaz Fontdevila, Ricardo Pommer, Rosita Smith (2009) Cumulus cell apoptosis changes with exposure to spermatozoa and pathologies involved in infertility. Fertility Sterility 91(5): 2061-2068.
- Matos L, Stevenson D, Gomes F, Silver-Carvalho JL, Almeida H (2009) Superoxide Dismutase expression in human cumulus oophorus cells. Mol Hum Reprod 15: 411-419.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...