Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1768
Research ArticleOpen Access
Effect of Transcranial Magnetic Stimulation and Cranial Electrotherapy Stimulation on Heroin Craving Volume 5 - Issue 4
Mostafa Shaaban Kandil1*, Wafik Said Bahnasy2, Amr Adel Haiba3, Gamal Taha Shamma4 and El-Sayed Abdel Hamid Gad5
- 1Assistant Lecturer of neuropsychiatry, Faculty of Medicine, Tanta University, Egypt
- 2Professor of Neuropsychiatry, Faculty of Medicine, Tanta University, Egypt
- 3Assistant Professor of Neuropsychiatry, Faculty of Medicine, Tanta University, Egypt
- 4Professor of Neuropsychiatry, Faculty of Medicine, Tanta University, Egypt
- 5Professor of Neuropsychiatry, Faculty of Medicine, Tanta University, Egypt
Received:August 03, 2021 Published:August 11, 2021
Corresponding author:Mostafa Shaaban Kandil, Assistant Lecturer of neuropsychiatry, Faculty of Medicine, Tanta University, Egypt
DOI: 10.32474/SJPBS.2021.05.000218
Abstract
Background: Craving is a central aspect of Heroin addiction and is considered a main cause of relapse after period of abstinence. The objectives of this work were to assess the efficacy of transcranial magnetic stimulation (TMS) and cranial electrotherapy stimulation (CES) on craving of heroin use disorder.
Methods: This study was performed on 80 patients with heroin use disorder. Patients were randomized either to receive active TMS versus sham TMS or to receive active CES versus sham CES. Cue induced heroin craving was assessed subjectively by brief substance craving scale (BSCS) and objectively by electroencephalogram (EEG) over 1 and 3 months follow up.
Results: The mean age of participants was 24.8 years and all of them were male. The study showed significant reduction of BSCS score and reduction of beta wave power after application of 10 sessions TMS or CES with least values in active TMS group which indicate less craving.
Conclusion: TMS and CES are effective non-invasive treatment modalities for the acute reduction of heroin craving Keywords: Craving; Heroin; Addiction; TMS; CES; EEG; BSCS
Introduction
Addiction is defined as a chronic relapsing neurobiological brain disease. The rate of drug addiction in Egypt is twice the global rate so working on the development of new preventive and treatment modalities is crucial. One of the most prevalent features of opioid addiction is the tendency to relapse on the drug even weeks, months or years after stoppage of opioid use. Exposures to stress or conditioned cues related to heroin abuse are distinct conditions that induce relapse. Craving (the intense desire to take a drug) is a central aspect of drug addiction and a contributing factor in relapse after period of abstinence. The National Institute on Drug Abuse reports that 40 to 60 % of people treated for substance use disorders relapse. For heroin, the numbers tend to be approximately twice that rate, 59% of which occurred within 1 week of discharge [1].
Aim of the Work
The aim of this work was to assess the efficacy of transcranial magnetic stimulation (TMS) and cranial electrotherapy stimulation (CES) on craving of heroin use disorder.
Subjects and Methods
The design of the current research was randomized controlled clinical trial to assess the effectiveness and outcome of Transcranial Magnetic Stimulation (TMS) and Cranial Electrotherapy Stimulation (CES) on heroin abuse craving. This study was done in Neuropsychiatry Department and Psychiatry and Neurology Center, Tanta University over a period of 27 months started from June 2018 through September 2020 on eighty patients who fulfilled the diagnostic criteria of heroin use disorder according to DSM-5.
Inclusion criteria consisted of heroin abusers aged 18-30 years. Exclusion criteria included patients with current medications which may alter EEG activity as anticonvulsants or patients with history of any neurological disorder that would result in abnormal EEG activity or current substance abuse other than heroin and the presence of implanted devices as cardiac pacemaker. Patients were randomized either to receive active TMS (20 patients) versus sham TMS (20 patients) or to receive active CES (20 patients) versus sham CES (20 patients). All patients were subjected to history taking included personal or drug history, systemic or mental state examination and urine drug screen. Craving was induced by cues of heroin blocks or pictures before applying the questionnaire or performing the EEG. Craving was assessed objectively by EEG or subjectively by BSCS at base line before starting TMS or CES, then at the next day after completion of 10 session of TMS or CES and after one or three months follow up.
For EEG recordings, participants were asked to close their eyes and to avoid mental activities as well as movements or muscular contractions during the recordings. EEG was recorded with Neurofax nihon Kohden QP-110 AK from scalp locations placed according to 10-20 international system. Resting EEG was recorded for 5 min to identify the baseline background activity of each patient. The EEG was digitized, and fast Fourier transformation (FFT) was performed. Fourier analysis converts a signal from its original domain (often space or time) to a representation in the frequency domain and vice versa. To calculate EEG power, the frequency spectrum was divided into 0.2 Hz bands and collapsed into EEG frequency bands of delta (1- 3.9 Hz), theta (4.0-7.9 Hz), alpha (8.0-12.9 Hz) and beta (13.0- 30 Hz). Each power value represented 5 seconds, and we analyzed 30 seconds of recording per case. Then we designated these power values as average percentages of total power. These were usually called delta, theta, alpha, and beta power ratios. Then, we exposed each patient to cue-induced craving pictures or previous heroin use situations according to each patient`s history of intake for recording a baseline EEG. Instructions were to sit relaxed, still and to carefully attend to the cues without employing distracting thoughts. The frequency domain analysis was performed using the Fast Fourier Transform (FFT) algorithm to calculate absolute (μV2/Hz) power density, relative (%) power density and mean frequency (Hz) within each of the sub-bands. The absolute power of a band is the integral of all of the power values within its frequency range. Relative power (RP) indices for each band were derived by expressing absolute power in each frequency band as a percent of the absolute power (AP) summed over the four frequency bands. Absolute power was log transformed (log x) and, relative power variables were transformed by log {x/1-x} in order to normalize the distribution of the data. EEG frequency (Hz) indices were found to be normally distributed and thus did not require transformation [2]. The BSCS is a self-report instrument to assess craving for heroin abuse over a 24-hour period. Patients were asked to complete a scale, rating the intensity, frequency and length of their cravings. Ratings were then scored on a scale of 0 to 12 (0 meaning no cravings; 12 meaning the patient was experiencing severe cravings) [3].
TMS Protocol
Group I patients (40 patients) received TMS sessions after one week of heroin abstinence. Before starting the study, individual TMS motor thresholds were determined for each participant. Individual motor threshold (MT) was determined similar to the method described [4]. TMS intensity was varied using an ascending staircase procedure and the motor evoked potential (MEP) of the abductor pollicis brevis muscle was assessed. High Frequency rTMS (HF rTMS) at 10 Hz (24 trains, 5 s per train, 25 s intertraininterval, i.e. 1200 pulses, 90% MT) was applied via a figure-eight coil with an outer winding of 70 mm connected to a Magstim Rapid-2 stimulator [5] targeting the left DLPFC. The duration of each session (real or sham TMS) will be 20 minutes for 5 days per week for 2 weeks, so each patient received 10 sessions. Sham stimulation was administrated at the same location, strength and frequency with the coil angled 45o away from the skull. This method reproduced sound and some somatic sensation (vibration and contraction of scalp muscles) that resemble active stimulation while generating intracerebral voltage approximately 1/3 that of active TMS stimulation [6].
CES Protocol
Group II patients (40 patients) received CES sessions after one week of heroin abstinence. Twenty-minute (5 days per week for 2 weeks) application of 10 sessions of CES using alphastim technology (Electromedical products international Inc., Mineral wells, Texas; www.alpha-stim.com ). Earclips electrodes were moistened with a conducting solution and attached to the earlobes. Current levels of the CES device (which range from 100 to 500 microamperes) were adjusted following manufacture recommendations to a comfortable level just below where vertigo is experienced. Sham CES was administrated at same location, strength and frequency but with CES device turned on and off. The collected data were organized, tabulated and statistically analyzed using SPSS version 19 (Statistical Package for Social Studies) created by IBM. There were descriptive and comparative types, where quantitative data were summarized as mean and standard deviations while qualitative data were summarized as numbers and percentages. A comparison was made using Paired student test (t - test) in case of two groups quantitative data. The chi-square test(X2) and Fisher exact test (FET) were used for qualitative data. Differences were considered significant if the P value was 0.05 or less. The study’s protocol was approved by The Research Ethics Committee and Quality Assurance Unit, Faculty of Medicine, Tanta University. Participations were voluntary, informed consents were obtained from all included patients and the possible risks were clarified.
Results
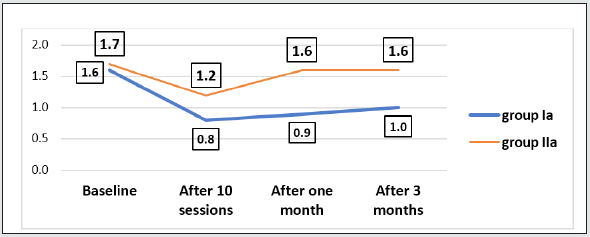
The current study showed that the mean age of patients was (24.8 ± 3.8) without significant difference among different study groups (Table 1). The participants in this study were all male (100%). Five heroin abuser females refused to participate in our study secondary to fear and stigma despite maintained privacy and confidentiality of the data. According to the marital status of the participants, half of them (50%) were single. Technical educational level was the commonest among participants. Most of participants were in the middle and low social class levels. According to the family characters, most families were of extended type. Tobacco abuse was the commonest substance of abuse by families followed by cannabis and heroin. Antisocial personality disorder represented the main personality disorder among participants. Peer influence (62.5%) and curiosity represented the main causes of heroin abuse between study group’s patients. According to addiction severity index, drug abuse was considered the main problem among this study participants followed by employment problems. Intravenous injection (63.7%) of heroin was the commonest form of heroin intake among all participants. There was no significance regarding the daily dose of heroin or the duration of abuse among the study groups. The current study showed no significant difference regarding the BSCS baseline scores between all study groups before performing any TMS or CES sessions. The current study showed significant decrease in BSCS values after (10 sessions of active TMS or active CES) and after one month and three months follow up with least values in active TMS group which indicate the least craving in active TMS group (Tables 2 & 3). After three months follow up there was slight increase in BSCS values which indicate more heroin craving. Despite these observed increase in BSCS values with follow up, the active TMS group subjects showed the least increase among all participants and still had significant difference with baseline craving (Figure 1). Power ratios are an index of EEG power reflecting changes in the balance of EEG power by frequency band. The current study showed no significant difference regarding mean log transformed beta band power at baseline before performing any TMS or CES sessions (Table 3). The current study showed significant decrease in mean log transformed beta band power values (after 10 sessions TMS or CES) and after one month follow up with least values in active TMS group which indicate less craving in this group (Figure 2). This study showed no significant difference between mean baseline beta band frequencies at F3 and F4 among study groups with higher values at F3 than F4 which indicated relative greater activation of left frontal hemisphere than the right one. The mean beta band frequency in active TMS group after performing 10 sessions TMS or after one month follow up at F3 was lower than F4 which correlated with the effectiveness of active TMS in modulation of brain activity in left prefrontal region (Table 4).
Figure 1: Comparison of the BSCS measures between the active TMS and active CES groups throughout the whole period of the study.
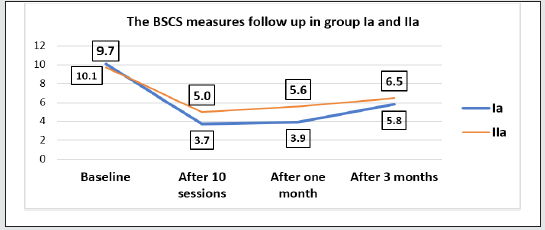
Figure 2: Comparison of the beta band absolute power between the active TMS and the active CES groups throughout the whole period of the study.
n: Number, SD: Standard deviation, %: Percentage
Table 2: Follow up of the Brief Substance Craving Scale measures in the active TMS group throughout the whole period of the study.
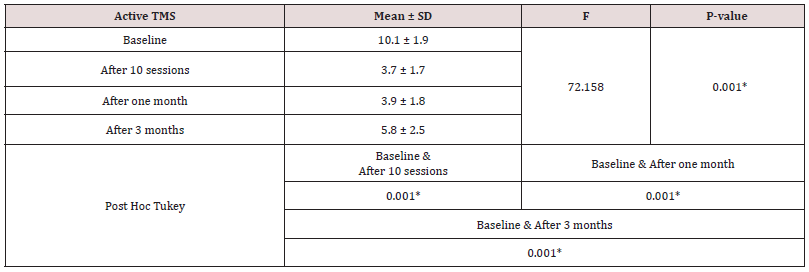
Table 3: Follow up of the Brief Substance Craving Scale measures in the active CES group throughout the whole period of the study.
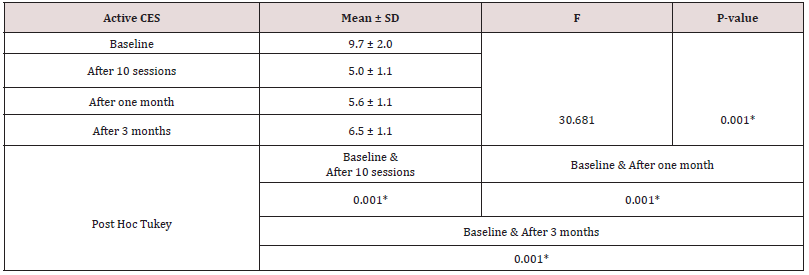
Table 4: The comparison between frequency of beta band at F3 and F4 after 10 sessions among study groups.

Discussion
Craving for heroin can be described as a powerful urge to use heroin again. Patients with craving have an intense desire to use heroin accompanied by vivid day dreaming about using heroin or difficulty in focusing on anything other than getting it. The current study showed that the mean age of patients was (24.8 ± 3.8) without significance among different study groups. The participants in this study were all male (100%). The higher ratio in heroin abuse in males may be attributable to the more freedom that males can obtain in our society and the easier ways to obtain the drugs than females. In Egypt, females still have lower prevalence rates than men due to culture effect. The involvement of 100% male gender in this research may provide an advantage of avoidance the genderassociated differences in craving as a result of hormonal changes throughout the menstrual cycle but it also lacking the advantage of gender difference studying [7]. The current study showed no significance regarding the BSCS baseline measures between all study groups but after 10 sessions TMS or CES these values were decreased with significant difference in active TMS and CES groups which indicate significant reduction of heroin craving but the maximum decrease was noticed in subjects who received active TMS sessions than active CES. So, both active TMS and active CES were significantly effective in reducing heroin craving but TMS was significantly more effective than CES. After three months follow up there was slight increase in BSCS values which indicate more heroin craving. Despite these observed increase in BSCS values with follow up, the active TMS group subjects showed the least increase among all participants and still had significant difference with baseline craving.
This downward shift of BSCS values after 10 sessions TMS or CES confirm the acute efficacy of these maneuvers to decrease craving in heroin use disorder patients especially in active TMS group but the observed slight increase of BSCS scores (which indicate more heroin craving) especially after three months follow up may confirm the need for further booster TMS or CES sessions throughout a longitudinal six month protocol. A prominent factor for the increase of craving within three months follow up is the presence of environmental drug cues. Continuous changes in reward and memory brain circuitry which are related to drug dependence result in high sensitivity to drug-linked cues during abstinence [8]. Furthermore, impairment in the regulation of the hypothalamic-pituitary-adrenal axis caused by opioid dependence is correlated to enhanced sensitivity to stressors during abstinence. Such abstinence related impairment of reward and stress response systems may explain the subjective experiences reported by study participants during abstinence and the constant desire for drugs even in case patients are in residence and living far from social, drug use-related, environmental stimuli of craving. Shi et al study corroborated our findings regarding a significant reduction of heroin craving among participants after one month follow up after abstinence [9]. On the contrary, Fathi et al declared a reduction of heroin craving for one month follow up but without statistical significance. Such a difference may arise from the different psychometric test used, small sample size and absence of drug cues [10].
According to Jack et al in a systematic review and meta-analysis regarding effect of rTMS on craving in substance dependence patients, rTMS revealed a significant anti-craving effect of the left DLPFC in patients with substance dependence. Excitatory rTMS targeting left DLPFC shows promise in reducing both craving and substance consumption, which may be a result of dopamine release and/or activation of the dorsal PFC executive functioning system. However, this effect was limited in duration, as indicated by a nonsignificant treatment effect at follow-up [11]. CES may produce its effect on decreasing heroin craving with ear-clip electrodes through achieving parasympathetic nervous system dominance by stimulating the auricular branch of the vagus nerve. Withdrawal symptoms including craving are basically manifestations of sympathetic nervous system over activity. CES also improves anxiety and frequent insomnia which are common symptoms in the early stage of recovery from heroin and these symptoms are the main precursor for relapse. In our current study, these effects appear to be session linked with increased craving or desire to take a heroin again after stoppage of CES sessions especially with continued follow up for three months. The current study showed that the mean log transformed beta band power values at base line had no significant difference between the study groups but after 10 sessions TMS or CES these values were decreased with significant difference in both active TMS or active CES study groups which indicate less craving but the maximum decrease was noticed in subjects who received active TMS sessions. So, both active TMS and active CES were significantly effective in reducing heroin craving but TMS was significantly more effective than CES. Active TMS group showed significant decrease of absolute beta band power values from baseline to 10 sessions application or after one or three months follow up. These results were in agreement with Jurgen et al who showed that absolute beta power significantly decreased with rTMS sessions but this study results were related only to the acute effects of TMS without further follow up. Jurgen reported that absolute beta band power was significantly decreased more with active TMS versus sham TMS. He postulated that active TMS session might mimic craving action on brain reward functions producing an increase of dopamine release with further cognitive inhibitory control mechanism [12].
Our study showed no significant difference between mean baseline beta band frequencies at F3 and F4 among study groups with higher values at F3 than F4 which indicated relative greater activation of left frontal hemisphere than the right one. The mean beta band frequency in active TMS group after performing 10 sessions TMS and after one and three months follow up at F3 was lower than F4 which correlated with the effectiveness of active TMS in modulation of brain activity in left prefrontal region. We reported that the left frontal hemisphere had greater activation than the right hemisphere after cue induction and therefor the choice of left DLPFC for neuromodulation of substance induced craving is an optimum location to manipulate. Our findings were in agreement [3,5].
Conclusion
Transcranial magnetic stimulation (TMS) and cranial electrotherapy stimulation (CES) are effective non-invasive treatment modalities for the acute reduction of heroin craving with significant reduction of heroin craving through three months after heroin abstinence. So, TMS or CES are considered valuable noninvasive devices to overcome the risky period of heroin craving especially after one month of heroin abstinence. So, TMS or CES represented new effective method for relapse prevention in heroin use disorder. Active TMS shows significant difference for reduction of heroin craving than sham TMS. Active TMS is more effective treatment modality for reduction of heroin craving than active CES. The observed slight increase of BSCS scores or the beta band power readings (which indicate more heroin craving) especially after three months follow up may confirm the need for further booster TMS or CES sessions throughout a longitudinal six-month protocol. Despite this observed slight increase of BSCS scores or the beta band power within three months after abstinence, the BSCS scores or the beta band power still have significant difference than the baseline readings. The left frontal hemisphere has greater activation than the right hemisphere after cue induction. Therefore, the choice of left DLPFC for neuromodulation of substance induced craving is an optimum location to manipulate
Recommendations
We recommend the use of rTMS or CES as non-invasive treatment options for acute reduction of heroin craving which provide improvement in the adherence to the recovery programs and prevention of relapse. We recommend the use of TMS for acute reduction of heroin craving as a superior efficient tool than CES. However, CES provides an easily accessible home treatment option for reduction of heroin craving especially if TMS apparatus is not available or if there are any contraindications of TMS use. We recommend the need to adopt a uniform prolonged treatment protocol and optimize the number of rTMS sessions in relation to treatment of patients with heroin use disorder during the whole abstinence months. We recommend the restrict prevention of heroin abuse patients during the abstinence period from exposure to heroin cues which induce craving that represents an important cause for heroin relapse. Violent craving can erupt suddenly into consciousness after exposure to drug cues, acting as a persistent cause for relapse. We recommend the presence of comprehensive effective rehabilitation program for six months for heroin abusers till complete normalization of brain neurobiological changes associated with heroin abuse.
Limitations
The need for further studies with longer follows up duration (six months) which may offer a more informative data about neurobiological changes for the whole abstinence period in heroin use disorder. The need for further studies to identify the neurobiological changes for heroin craving in females for proper assessment of the gender difference.
Declaration
Ethics approval and consent to participate section
- The manuscript was approved from The Research Ethics Committee and Quality Assurance Unit, Faculty of Medicine, Tanta University.
- The study’s protocol had permitted by The Research Ethics Committee and Quality Assurance Unit, Faculty of Medicine, Tanta University. Participations were voluntary, informed consents were approved by all participants’’ guardian and any possible risks were clarified.
Consent of publication: Not applicable.
Competing interests: All authors disclose that they have no competing interests related to the study.
Availability of data and materials: The datasets used and/ or analyzed during the current study are available from the corresponding author on reasonable request.
Authors Contributions:
MSK: participated in the study’s design, patients’ selection, statistical analysis, data analysis, references collection, manuscript writing and revision and final approval, WSB: participated in the study’s design, patients’ selection, EEG interpretation, statistical analysis, data analysis, manuscript writing, revision and final approval, AAH: participated in patients’ assessment and inclusion, data analysis, psychometric scale analysis, statistical analysis, references collection, manuscript writing, revision and final approval, GTS: participated in the study’s design, patients’ selection, and evaluation, data analysis, references collection. EAG: participated in study’s idea and design, patients’ assessment and inclusion, data analysis, references collection, manuscript writing, revision and final approval.
Acknowledgement
We would like to thank the neuropsychiatry department and Psychiatry and Neurology center medical and para-medical staff, Tanta University for their great help in patients’ selection and evaluation.
References
- Chotas JR, Bourne PE (1979) Heuristic techniques in the quantification of the electroencephalogram in renal failure. Comput Biomed Res 12(4): 299-312.
- Fathi H, Yoonessi A, Rezaei A, Majdzadeh R, Rezaeitalab F (2020) Effects of abstinence from opioids on self-reported craving and sleep. Cogent Psychology 7(1): 171- 344.
- Jack J, Kenneth N, Rang O, Andrew M, Georg S (2019) Effects of repetitive transcranial magnetic stimulation (rTMS) on craving and substance consumption in patients with substance dependence: a systematic review and meta-analysis. Addiction 114(12): 2137-2149.
- Judy L, Rebecca S, Niels J, Martijn F, Damiaan D (2019) Efficacy of Invasive and Non-Invasive Brain Modulation Interventions for Addiction. Neuropsychol Rev 29: 116-138.
- Jürgen P, Livia T, Igor R, Claus L (2014) Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex Decreases Cue-induced Nicotine Craving and EEG Delta Power. Brain Stimul 7: 226-233.
- Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA (2001) Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry 49(5): 460-463.
- Nicolas C, Russell T, Pierce A, Maldera S, Holley A, et al. (2019) Incubation of cocaine craving after intermittent-access self-administration: Sex differences and estrous cycle. Biol Psychiatry 85(11): 915-924.
- Schutter DJ, Van Honk J (2006) A standardized motor threshold estimation procedure for transcranial magnetic stimulation research. J ECT 22(3): 176-178.
- Sensen S, Anna Z, Wenjun G, Hui L, Xiaolin Z (2019) Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: A meta-analysis. Brain Stimul 12(3): 606- 618.
- Shi J, Li S, Zhang X, Wang X, Foll B, et al. (2009) Time-dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. Am J Drug Alcohol Abuse 35(5): 267-272.
- Smyth BP, Barry J, Keenan E, Ducray K (2010) Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J 103(6): 176-179.
- Somoza E, Dyrenforth S, Goldsmith J, Mezinskis J, Cohen M (1995) In search of a universal drug craving scale. Paper presented at the Annual Meeting of the American Psychiatric Association, Miami Florida, USA.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...