
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1768
Research ArticleOpen Access
Comparation Of Two Immunomagnetic Nanobeads In PIG-A Gene Mutation Assay In Vitro Volume 5 - Issue 5
Yi Zhang, Jiaqi Lv, Xiao Chen, Yulu Li, Haitao Yang, Qing Miao, Baolier Wuhan, Jingwei Xiao* and Binli*
- National Institute of Occupational Health and Poison Control, CHINA CDC
Received:September 06, 2021; Published:September 21, 2021
Corresponding author: Jingwei Xiao, Binli, National Institute of Occupational Health and Poison Control, CHINA CDC
DOI: 10.32474/SJPBS.2021.05.000223
Abstract
Objective: The enrichment ability was compared between two kinds of immunomagnetic nanobeads in phosphatidylinositol
glycans A (PIG-A) gene mutation assay.
Methods: Glycosylphosphatidylinositol(GPI)(+) TK6 cells was enriched and GPI (-) TK6 cells were removed by both PE and
Biotin immunomagnetic nanobeads. Flow cytometry was used to detect the purification effect. PIG-A gene mutation assay was
performed on purified TK6cells with typical positive Ethyl methane Sulfonate (EMS).
Results: After once purification, PE immunomagnetic nanobeads could reduce the GPI(-)background value from 44.90±1.63%
to 0.27±0.12%, while the value of Biotin immunomagnetic nanobeads was 4.27±0.76%.Compared with the control group ,the
mutation frequency of PIG-A gene increased significantly at 50μg/mL on the 12th day, at 100μg/mL and 200μg/mL on the 4th, 8th
and 12th day.
Conclusions: PE immunomagnetic nanobeadswere more suitable than Biotin immunomagnetic nanobeads for TK6 cells
purification of PIG-A gene mutation assay in vitro.
Keywords: PE Immunomagnetic Nanobeads; Biotin Immunomagnetic Nanobeads; PIG-A Gene Mutation; EM; Flow Cytometry
Abbreviations: EMS:Ethylmethane Sulfonate; PIG-A: phosphatidylinositol glycans A; GPI:glycosylphosphatidylinositol; FBS:Fetal Bovine Serum; PBS:Phosphate Buffer Solution.
Introduction
Genetic toxicology assessment is an important part of the safety
assessment of chemicals and pesticide products, and in-vitro model
research instead of animal experiments has become an important
direction of toxicology development [1]. In recent years, some new
genotoxicity tests have been developed and gradually applied to
toxicological safety assessment. Gene mutation analysis of PIG-A is
a new genotoxicity detection method based on somatic cell gene
mutation. This method saves manpower and material resources,
and is more efficient and quick [2].
PIG-A gene encodes to form GPI, which is the catalytic subunit
of N- acetylglucosamine transferase needed for biosynthesis in
ankyrin. It is highly conserved in structure and function among
different species. PIG-A gene is located on X chromosome, and
a single mutation in its fragment can affect the synthesis of GPI
anchor and lead to the deletion of GPI anchor chain protein on the
cell surface (i.e., GPI(-)).Therefore, the potential genotoxicity risk
of the test object can be evaluated by detecting the expression
level of the anchor chain protein (such as CD55 and CD59) on the
cell membrane surface [3-8]. In this study [9, 10], the purification
effects of two different immunomagnetic nanobeads on TK6 cells
were compared, and a simple and efficient cell purification method
was explored. The comparison results can be used as a technical
reference for PIG-A mutation assay in vitro.
Materials and Methods
Study design
TK6 cells were cultured normally, and GPI (+)TK6 cells were selected by immunomagnetic nanobeads. The results of GPI (-)TK6 cells after purification of two different kinds of immunomagnetic nanobeads were compared. The purified cells were poisoned by EMS, and the mutation of PIG-A gene was detected by flow cytometry.
Main materials and instruments
Human lymphoblastoid cells Tk6 and RPMI-1640 were purchased from American ATCC (American Type Culture Collection). Fetal Bovine Serum(FBS),Penicillin-Streptomycin Solution and Phosphate Buffer Solution(PBS) were purchased from Gibco Company. Ethyl methane Sulfonate (EMS,CAS No:62- 50-0) was purchased from Sigma company. APC mouse antihuman CD19 antibody (No.302212), PE mouse anti-human CD55 antibody (No.311308), PE mouse anti-human CD59 antibody (No.304708), 7-AAD Viability Staining Solution (No.420404), Biotin anti-human CD55(No.311304), MojoSort™ Human anti-PE Nanobeads(No.480092), MojoSort™ Biotin-Streptavidin Nanobeads (No.480015), MojoSort™ Magnet (No.480019), MojoSort™ Buffer (5X) (No.480017), and Human TruStain FcX™ (No.422301) were all purchased from bio legend Company of the United States.
Cell culture
TK6 cells were cultured in RPMI-1640 medium with 10% heatinactivated FBS and 1% penicillin-streptomycin solution. Cells were grown at 37℃ and 5% CO2 in a humidified incubator and were maintained between 0.2×106 and 1.0×106 cells/mL.
Enrichment of immunomagnetic nanobeads
PE immunomagnetic nanobeads and Biotin immunomagnetic nanobeads were used to enrich GPI(+) TK6 cells and remove pre-existing GPI(-)TK6 cells. MojoSort™ buffer was diluted to 1× concentration using double distilled water. After the cells were washed with PBS, they were resuspended with 1 mLMojosort buffer (the buffer should be placed on ice during all operations). Cells were counted and adjusted to 1×108 cells/ml. 100μLcells and 5μLHuman TruStain FcX™ were put into 5mLflow tube mixed well and incubated at room temperature for 10min. These paration procedure of the two kinds of immunomagnetic nanobeads were similar as follows: 5μLCD55-PE antibody or Biotin-CD55 antibody was added to the cell suspension, mixed well and incubated on ice for 15min. Add 4mL Mojo Sort™ buffer to wash cells, and centrifuge at 300×g for 5 min. The supernatant was discarded, and the cells were resuspended in 100μLMojoSort™ buffer. The magnetic nanobeads were vortexed for 5 times, and 5μL of Human anti-PE Nanobeads or Mojo Sort™ Biotin-Streptavidin Nanobeads were added to the cell suspension, mixed evenly, and incubated on ice for 15min. The cells were washed by adding 4 mL Mojosort buffer and centrifuged at 300×g for 5 minutes. The supernatant was discarded and 2.5mlMojoSort™ buffer was added. The flow tube was placed on the magnetic track for 5min, the unlabeled parts were discarded, and then the labeled cells were resuspended with 2.5mlMojoSort™ buffer. After repeating the above track separation step twice, add 2mL of culture medium to resuspend, and transfer to culture flask.
Ems Treatment
The purified TK6 cells were poisoned by EMS (0.5%DMSO was added for solubilization), and the cells were cultured in T25 culture flask with the density between 2×105and 5×105 cells/ml, and the poisoning concentrations were 50μg/mL, 100μg/mL and 200μg/ mL, for 24h. After poisoning, the cells were washed with PBS, and the culture mediums were changed.The cells were cultured normally, and the GPI(-)TK6 cells were detected on the 4th, 8th and 12th day after exposure.
Flow Cytometry Analysis
TK6 cells were washed with PBS and adjusted the cell density to 1×106 cells/ml, prepare a corresponding number of 2mLEP tubes, add 100μl cell suspension and 2.5μLHuman TruStain FcX™ into each tube, vortex and shake well, and incubated at room temperature for 10min. The CD19-APC,CD59- PE,7-AAD single staining tubes, and PE and APC isotype control tubes (antibody dosage: 2.5μL) were respectively established to adjust the fluorescence channel compensation of the flow cytometer. Determine the position of the gate and establish the detection template. Pure cells were used as negative control tubes. 2.5μlCD19-APC and 2.5μLCD59- PE antibody were added into the sample tubes of the exposed group and the control group, respectively. They were mixed uniformly by vortex oscillation, and incubated for 20 minutes on ice in the dark. The cells were washed with 2mlPBS, centrifuged at 350×g for 5min, and the supernatant was discarded (washed twice). The stained cells were resuspended in 0.5mLPBS, and 2.5μL7-AAD was added to each tube. After incubation for 5min in the dark on ice, the cells were detected and analyzed.
Statistical Analysis
SPSS22.0 software was used for statistical analysis; Shapiro- Wilk was used for normality test; Bartlett test was used for homogeneity test of variance; the data with normal distribution and homogeneous variance were analyzed by one-way ANOVA; when one-way ANOVA was statistically significant, LSD method was used for pairwise comparison between groups; The data that did not conform to normal distribution were analyzed by Kruskal- Wallis method. Inspection level α=0.05.
Results
The difference between two nanobeads
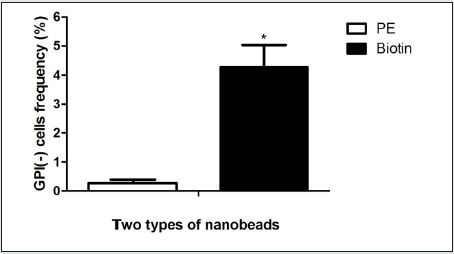
The gate of the target cell population was determined by using the flow cytometry detection template (Figure 1 A). GPI(+) TK6cells were located in Q1-UR while GPI(-)TK6cells were located in Q1-LR due to the absence of the GPI anchored protein CD 59 in wild-type TK6 cells (Figure 1B).The purification and screening effects ofTK6 cells purified by PE immunomagnetic nanobeads (Figure 1C) and purified by Biotin immunomagnetic nanobeads (Figure 1D) were analyzed respectively. The GPI(-)background value of wild-type TK6 cells was 44.90±1.63%.After once purification, PE immunomagnetic nanobeads could reduce the background value to 0.27±0.12%, and Biotin immunomagnetic nanobeads could reduce the background value to 4.27±0.76%.The difference was statistically significant (Figure 2), which shown that PE immunomagnetic nanobeads had better purification effect.
Figure 1A: Purification of TK6 cells by PE and Biontin immunomagnetic nanobeads. TK6 cells were analyzed by flow cytometry, and the target cells were in the P3 gate.
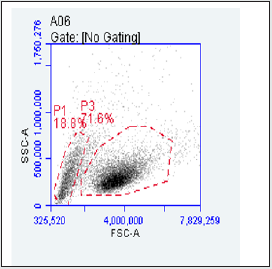
Figure 1B: Purification of TK6 cells by PE and Biontin immunomagnetic nanobeads. TK6 cells without purification,GPI(+) in theQ1-UR as well as GPI(-) in theQ1-LR.
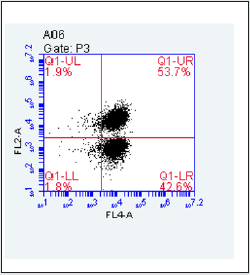
Figure 1C: Purification of TK6 cells by PE and Biontin immunomagnetic nanobeads. The fourth quadrant Q1-LR was the frequency of GPI(-) cells after purification of TK6 cells by PE immunomagnetic nanobeads.
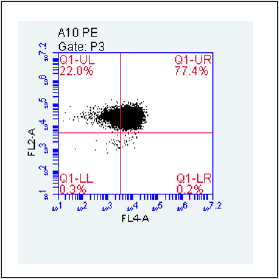
Figure 1D: Purification of TK6 cells by PE and Biontin immunomagnetic nanobeads. he fourth quadrant Q1-LRwas the frequency of GPI(-) cells after purification of TK6 cells by Biotin immunomagnetic nanobeads.
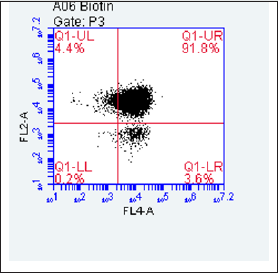
Figure 2: Comparison of GPI(-) cells purified by two kinds of immunomagnetic nanobeads. (* Compared with PE immunomagnetic nanobeads, the purification effect was P<0.05)
Results of PIG-A gene mutation assay
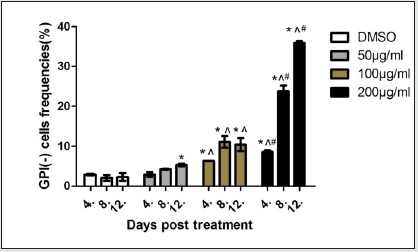
As shown in Figure 3,compared with the control group, the mutation frequency of PIG-Agene increased significantly at 50μg/ mL group on the 12th day (P<0.05). Compared with the control group and 50μg/mL group, the mutation frequency of PIG-A gene increased significantly at 100μg/mL group on the 4th, 8th and 12th day (P<0.05). Compared with the other three dose groups, the mutation frequency of PIG-A gene increased significantly at 200μg/ mL group on the 4th, 8th and 12th day (P<0.05).
Figure 3: GPI(-) cell rate in different dose groups after EMS exposure. (* P<0.05 compared with control group, ^ p < 0.05 compared with 50μg/mL group, and#p < 0.05 compared with 100μg/mL group)
Discussion
In this study, human lymphoblastic TK6 cells were selected, which originated from human hematopoietic system.TK6 were suspended cells with complete p53 gene function, and had high sensitivity and specificity in genotoxicity test. Selecting the human cell line was helpful for risk assessment and extrapolation the results from in vitro test to human body [11]. There were GPI(-) pre-existing in TK6cells, in order to improve the sensitivity of detection test, it was necessary to remove GPI(-)from TK6 cells [12]. Immunomagnetic nanobeads are promising enrichment agents which has been widely used to separate and concentrate specific cells, microorganisms, protein and other substances [13-17]. The main principle is that the specific antibody on the surface and then combined with the corresponding antigen in the liquid state by the action of hydrodynamics. The target antigens are separated from other impurities through multiple magnetic separations, so as to obtain highly concentrated antigen [18,19]. In this study, CD55 was selected as the binding site of immunomagnetic nanobeads for cell screening, and the separation effects of anti-fluorescein immunomagnetic nanobeads andbiotinstreptavidin immunomagnetic nanobeads were compared. The comparison results showed that the purification effect of PE immunomagnetic nanobeads was better. Itmight be related to the different combination , but the specific mechanism needed to be studied. PE immunomagnetic nanobeads have the characteristics of high sorting efficiency and good specificity. It provides the necessary technical support for the study of PIG-A gene mutation assay in vitro. In this study, EMS was used as a typical positive substance to verify the mutation of PIG-A gene in TK6 cells purified by PE immunomagnetic nanobeads. Three time points and three exposure dose groups were selected to analyze the dose-time effect. It was found that the mutation frequency increased significantly at 100μg/mL and 200μg/mL groups on the 4th day after exposure, The shortest detection period stipulated in OECD490 TK gene mutation test of mammalian cells in vitro is at least 12-14 days [20], which shows that the PIG-A gene mutation assay based on flow cytometry with purified TK6 cells has high sensitivity. Compared with the existing gene mutation detection method, PIG-A gene mutation assay in vitro is simple in operation, fast in sample preparation, short in test period, and high-throughput detection by using flow cytometry. On the other hand, it can be used as a supplementary test of PIG-A gene mutation assay in vivo and an additional test of positive results of other mutagenic tests. It has the potential to be applied to screening and evaluating the genotoxicity of chemicals and pesticides.
Acknowledgement
Acknowledgement: Thanks to the National Key Research and Development Program of China,( No. 2017YFF0211201) for supporting this study
Disclosure of Interest
The authors declare that there is no conflict of interests.
References
- HUO Jiao, LI Yan, Chen Jinyao, Zhang Lishi (2015) Application and development of PIG-A gene mutation assay. Chin J Pharmacol Toxicol 29(1): 174-178.
- YU Chun rong, YUAN Tai chang, ZHOU Chang hui, HUANG Peng cheng,TONG Wen,et al. (2018) Application of PIG-A gene mutation in genotoxicity research. Chinese Journal of New Drugs 27(8): 892-898.
- Javier R Revollo, Azra Dad, Mason G Pearce, Roberta A Mittelstaedt, Andrea Casildo, et al. (2020) CD59-deficient bone marrow erythroid cells from rats treated with procarbazine and propyl-nitrosourea have mutations in the PIG-A gene. Environ Mol Mutagen 61(8): 797-806.
- Ann Karin Olsen, Stephen D Dertinger, Christopher T Kruger, Dag M Eide, Christine Instanes, et al. (2017) The PIG-A Gene Mutation Assay in Mice and Human Cells: A Review. Basic & Clinical Pharmacology &Toxicology 121(Suppl 3): 78-92.
- Gollapudi BB, Lynch AM, Heflich RH, et al. (2015) The in vivo pig-a assay: A report of the internationalworkshop on Genotoxicity testing (IWGT) workgroup. Mutat Res Genet Toxicol Environ Mutagen 783: 23-35.
- David R, Talbot E, Allen B, Amy W, Usman A, et al. (2018) The development of an in vitro pig-a assay in L5178Y cells. Arch Toxicol 92(4): 1609-1623.
- Bemis JC, Avlasevich SL, Labash C, McKinzie P, Revollo J, et al. (2018) Glycosylphosphatidylinositol (GPI) anchored protein deficiency serves as a reliable reporter of pig-a gene mutation: support from an in vitro assaybased on L5178Y/Tk(+/-) cells and the CD90.2 antigen. Environ Mol Mutagen 59(1): 18-29.
- Xu Tian, Youjun Chen, Jun Nakamura (2019) Development of a novel PIG-A gene mutation assay based on a GPI-anchored fluorescent protein sensor. Genes and Environment 41: 10-21.
- Christopher T, Krüger, Mareike Hofmann, Andrea Hartwig (2015) The in vitro PIG‑A gene mutation assay: mutagenicity testing via flow cytometry based on the glycosylphosphatidylinositol(GPI) status of TK6 cells. Arch Toxicol 89(12): 2429-2443.
- Benjamin J Rees, Matthew Tate, Anthony M Lynch, Catherine A Thornton, Gareth J Jenkins, et al. (2017) Development of an in vitro PIG-A gene mutation assay in human cells. Mutagenesis 32(2): 283-297.
- LI Ruowan, ZHOU Changhui, HUANG Pengcheng, CHANG Yan (2019) Establishment of an in vitro PIG-A gene mutation assay based on TK6 cells for genotoxicity tests. Carcinogenesis, Teratogenesis & Mutagenesis 31(3): 242-248.
- Christopher T Krüger, Bettina M Fischer, Olivier Armant, Volker Morath, Uwe Strähle, et al. (2016) The in vitro PIG‑A gene mutation assay: glycosylphosphatidylinositol (GPI)‑related genotype‑to‑phenotype relationship in TK6 cells. Arch Toxicol 90(7): 1729-1736.
- Shore DE, Dileepan T, Modiano JF, Jenkins MK, Stadler BJH (2018) Enrichment and Quantification of Epitope-Specific CD4+ T Lymphocytes Using Ferromagnetic Iron-Gold and Nickel Nanowires. Scientific Reports 8: 15696.
- Jeon YS, Shin HM, Kim YJ, Nam DY, Park BC, et al. (2019) Fe-Au Barcode Nanowires as a Simultaneous T Cell Capturing and Cytokine Sensing Platform for Immunoassay at the Single-Cell Level. ACS Appl Mater Interfaces 11: 23901-23908.
- Bhana S, Wang Y, Huang X (2015) Nanotechnology for Enrichment and Detection of Circulating Tumor Cells. Nanomedicine (Lond) 10(12): 1973-1990.
- Nemati Z, Um J, Zamani Kouhpanji MR, Zhou F, Gage T, Shore D, et al. (2020) Magnetic Isolation of Cancer-Derived Exosomes Using Fe/Au Magnetic Nanowires. ACS Appl. Nano Mater 3: 2058-2069.
- Sharma A, Orlowski GM, Zhu Y, Shore D, Kim SY, et al. (2015) Inducing Cells to Disperse Nickel Nanowires via Integrin-Mediated Responses. Nanotechnology 26(13): 135102.
- Wen Yiming, Xu Jinting, Xiang junjian (2013) Advances in immunomagnetic beads enrichment technology. Chinese Journal of Immunology 29: 88-92.
- Mohammad Reza Zamani Kouhpanji, Bethanie JH Stadler (2020) A Guideline for Effectively Synthesizing and Characterizing Magnetic Nanoparticles for Advancing Nanobiotechnology: A Review. Sensors 20(9): 2554.
- Oecd Guideline For The Testing Of Chemicals (2016) In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene,OECD490.OECD Council.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




