
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4722
Research Article(ISSN: 2637-4722) 
Tree Nut and Peanut Allergy in a Portuguese Pediatric Cohort- Clinical Characterization and Anaphylaxis Predictors Volume 4 - Issue 4
Leonor Esteves Caldeira1*, Rita Brás1, Cláudia Varandas1, Fátima Cabral Duarte1, and Célia Costa1,2
- 1Immunoallergology Department, Hospital de Santa Maria, Centro Hospitalar Universitário Lisboa Norte EPE, Portugal
- 2Clínica Universitária de Imunoalergologia, Faculdade de Medicina, Universidade de Lisboa, Portugal
Received: September 05, 2023 Published: September 15, 2023
Corresponding author: Leonor Esteves Caldeira, Immunoallergology Department, Hospital de Santa Maria, Centro Hospitalar Universitário Lisboa Norte, Portugal
DOI: 10.32474/PAPN.2023.04.000193
Abstract
Background: Tree nuts and peanuts (TN/P) are frequent causes of anaphylaxis in children. Our aim was to characterize a Portuguese pediatric cohort with TN/P allergy and to assess skin tests (ST), specific IgE (sIgE) and molecular components (mcIgE), as well as sIgE/total IgE ratio’s utility in anaphylaxis prediction.
Methods: Retrospective study (2017-2021) of pediatric patients with TN/P allergy, grouped according to reaction severity (group 1-anaphylaxis vs group 2-milder reaction). ST mean papule diameter (MPD), sIgE (ImmunoCAP®), mcIgE (ISAC®) and sIgE/total IgE ratio were compared (SPSS®, p<0.05: statistically significant).
Results: A total of 98 patients were included, 64% male. 88% had concomitant allergic disease and 40% had allergy family history. The more common culprit nuts were peanut (47%), walnut (31%) and hazelnut (13%). Index reaction symptoms were mostly cutaneous (46%), followed by anaphylaxis (36%). Chestnut and cashew sensitization were significantly associated with anaphylaxis (OR=5.023, p=0.002; OR=2.901, p=0.018). MPD was higher in G1 for almond, cashew, and pistachio (p<0.05). sIgE was not a good severity predictor for any TN/P, however, a significantly higher sIgE/total IgE ratio was found in G1 for walnut (p=0.023). McIgE was obtained in 49%: peanut Ara h2 and Ara h6 were more represented in G1 (2.8 vs 0 ISU-E, p=0.042; 1.3 vs 0 ISU-E, p=0.020).
Conclusion: Peanut, hazelnut and walnut were the most frequent nuts involved in TN/P allergy. Anaphylaxis was the first manifestation in 36% of the patients, significantly more frequent in chestnut and cashew allergic children. The authors suggest that MPD should be valued not only for diagnosis, but also for anaphylaxis risk prediction in almond, cashew, and pistachio allergic patients. sIgE/total IgE ratio seems to be useful in anaphylaxis prediction.
Keywords: Anaphylaxis; component-resolved diagnosis; food allergy; peanut allergy; sensitization profile; tree nut allergy
Impact Statement: The present study characterizes a Portuguese cohort of 98 pediatric patients with tree nut and peanut allergy, pointing out the clinical utility of skin tests, specific IgE and molecular components in anaphylaxis prediction, as well as the interesting adding value of sIgE/total IgE ratio for nut allergy in clinical practice.
Introduction
Tree nut (TN) and peanut allergy prevalence has been increasing over the last 20 years, particularly in pediatric age, as a presumable result of changes in recent eating habits [1-3]. Allergy prevalence for each TN appears to vary in different parts of the world. A current systematic worldwide review of studies estimated the global prevalence of probable TN allergy to range from 0.05% to 4.9%, and peanut allergy between 0.5% and 2.5% [4,5]. These food allergies can be potentially life-threatening, accounting for a high number of fatal food-induced anaphylaxis, even when ingested in very small quantities or inadvertently, as occult allergens. Recent studies reported TN and peanut allergies as the responsible for 70-90% of deaths from food-induced anaphylaxis, with TN alone accounting for 18-40% [6]. TN and peanut allergies usually develop early in life and tend to persist into adulthood. According to previous published data, acquisition of natural tolerance to TN and peanut occurs in only 9%-20% of allergic patients [7-9].
The constant need for caution when choosing food and the potential risk of anaphylaxis, frequently leading to diet and social activities restrictions, significantly affects both patient and family’s quality of life. Presently, and regardless of years of research, the management cornerstone of these patients remains strict avoidance of the incriminated nut, in addition to patient and family’s education on prompt recognition of anaphylaxis and immediate use of adrenaline [10,11]. Other treatment possibilities have been largely explored, namely oral immunotherapy, for peanut and TN allergic patients, but their use is still limited [12]. Homology amongst nut proteins and cross-reactivity between their main allergens (namely 2S albumins, 7S and 11S globulins, lipid transport proteins [LTPs], and PR-10) leads to frequent co-sensitization in nut allergic patients, which does not always mean a true concurrent allergy to different nuts.
As a result, it can be challenging to manage these patients and a distinction between cross-sensitization and clinically relevant cross-reactivity between different TN and peanut is critical, although it frequently requires multiple oral food challenges (OFC) with the associated risk of a possible anaphylaxis. For this fact, dietary restriction of all TN and peanut is a common practice. Deeper knowledge of sensitization patterns and investigation of possible anaphylaxis predictors would be of great value to establish a more precise diagnostic approach and individual dietary guidance for patients allergic to these foods. We aimed to characterize a pediatric cohort with TN and/or peanut allergy followed in an Immuno allergology department of a Portuguese tertiary hospital, and to assess the utility of skin tests (ST), specific IgE (sIgE) and molecular components (mcIgE), as well as the ratio sIgE/total IgE, in predicting the anaphylaxis risk [13].
Methods
Study design and population
A five-year single-center, observational, retrospective study was conducted, including Portuguese children (0-18 years-old) referred to Food Allergy consultation for suspected IgE-mediated TN and/or peanut allergy between January 2017 and December 2021. The study was approved by the hospital’s ethics committee and all patients’ parents/guardians gave an informed consent.
Data collection and Interventions
Patients’ charts were reviewed for demographic, clinical and analytical data collection. Detailed information for the characterization of the index reaction (first nut reaction which motivated referral to Food Allergy consultation) was obtained. Post-index nut reactions or nut allergies diagnosed during patients’ allergological investigation were also assessed. Patients were considered allergic to a specific nut if they presented a suggestive clinical history and at least one positive test of the following: (1) skin prick/prick-prick tests (SPT/SPPT); (2) sIgE; (3) OFC. In the absence of previous contact or in cases of previous ingestion without reaction, and evidence of sensitization to a specific nut (positive SPT/SPPT and/or sIgE), only those with a positive OFC were considered allergic.
Previous personal and family history of atopy was also recorded. Allergological investigation included ST, serum analysis of total IgE, sIgE for TN, peanut and mcIgE, as well as OFC. ST was considered positive if mean papule diameter (MPD) ≥3mm larger than negative control. When SPT was negative or commercial extract was unavailable, SPPT was performed. Regarding serum analysis, cut-off value for positive result for sIgE (ImmunoCAP®, Thermo Fisher Scientific) was >0.35 kU/L, while for mcIgE (ImmunoCAP® ISAC-112, Thermo Fisher Scientific) was ≥0.3 ISU-E [14,15]. OFC was performed only in selected cases, as it is the gold standard for allergy confirmation or exclusion. Selection criteria for OFC included patients with skin sensitization to non-culprit nuts, but with negative or low levels of sIgE (<2 kU/L) to the respective nut, and patients with negative skin tests/sIgE to a respective nut (non-culprit or culprit).
Anaphylaxis predictors assessment
Children were grouped according to reaction severity in group 1 (G1) if patients fulfilled anaphylaxis criteria, based on the definition of Muraro et al. [16], or group 2 (G2) if not - oral allergy syndrome or systemic reaction with only one of the following: involvement of the skin-mucosal tissue (e.g., generalized hives, itch-flush, swollen lips-tongue-uvula), respiratory compromise (e.g., dyspnea, wheezebronchospasm, stridor, reduced PEF, hypoxemia) or persistent gastrointestinal symptoms (e.g., crampy abdominal pain, vomiting). The following variables were compared between groups: MPD of SPPT (for methodologic uniformization only SPPT were considered in this analysis), sIgE and mcIgE values, as well as ratio sIgE/total IgE.
Statistical analysis
Continuous variables were presented as means and standard deviations (SD), or medians and interquartile ranges for variables with skewed distributions, and categorical variables as frequencies and percentages. Normal distribution was confirmed using the Shapiro-Wilk test. Categorical variables were compared using Fisher’s exact test or the Chi-square test, as appropriate, while t-independent test and Mann-Whitney test were used to compare parametric and non-parametric independent samples, respectively. P<0.05 was considered statistically significant. Analyses were performed with the use of IBM SPSS software (version 25.0).
Results
Clinical characterization
A total of 150 clinical records of children with suspected TN and/ or peanut allergy were reviewed, of which 52 were excluded (38 for exclusion of nut allergy and 14 for incomplete data). As a result, 98 patients were included in this study, of whom 63 were male (64%). The majority of patients (n=86, 88%) presented a concomitant allergic disorder: 76 (78%) had allergic rhino conjunctivitis, 50 (51%) had asthma, 45 (46%) had another food allergy, including 30 (31%) with egg allergy, and 43 (44%) had eczema. Thirty-nine (40%) patients had a family history of allergy (Table 1). Culprit nuts were peanut (n= 62, 63%), hazelnut (n=58, 59%), walnut (n=52, 53%), almond (n=37, 38%) and cashew (n=31, 32%), followed by chestnut (n=19, 19%), pistachio (n=18, 18%) and pine nut (n=6, 6%). Eighty-eight (86%) patients reported reaction to a singlenut, although only 31 (32%) were monosensitized after allergological investigation was carried out (16 to peanut, 7 to walnut, 4 to cashew, 2 to hazelnut and 2 to pine nut).
Index reaction
Patients’ mean age of index reaction was 5.8 years (SD 4.6, range 0.2-17), being significantly lower in patients describing peanut reactions (4.6 years (SD 3.5, range 0.2-17 years), comparing with patients describing TN reactions (6.7 years, SD 5.8, range 0.2-17 years) (p=0.02). In 96 (98%) patients, symptoms appeared within 30 minutes after exposure. The index reaction occurred before the age of 2 in 26% of patients and in the majority (59%) before the age of 5. Index reaction manifestations were more frequently cutaneous (n=45, 46%), with urticaria/angioedema in 38 (84%) children and eczema exacerbation in 7 (16%). Eczema exacerbation was confirmed in all patients with eviction diet followed by reintroduction with reproducible reaction (5 of them reintroduced the nut at home on their own initiative and photo-documented the reproducible lesions, and the remaining two performed oral challenge at the hospital). Thirty-five (36%) patients described a reaction compatible with anaphylaxis. Gastrointestinal symptoms were reported by 16 (16%) patients, of whom 9 had oral allergy syndrome, 5 nausea and vomiting and 2 abdominal pains. Lastly, 2 (2%) patients presented shortness of breath.
Skin tests and laboratory parameters
More than half of the allergic population (n=50, 51%) had positive SPT results to various nuts and 38 out of 52 patients who performed SPPT (73%) had positive results. MPD of SPT and SPPT to the respective nuts are represented in Table 2, as well as whole extract sIgE. Mean value of total IgE was 1860 U/mL (SD 1984, range 17-6314 U/mL). Molecular components were obtained for 48 (49%) patients. The most frequent allergens were from LTP family, predominantly Pru p 3, for which 20 (42%) patients showed positivity. Subsequently, the most relevant was Ara h 9 (n=17, 35%), followed by Jug r 3 (n=16, 33%), Cor a 8 (n=12, 25%) and Tri a 14 (n=4, 8%). The 2S albumin family was the second most documented, namely Jug r 1 (n=18, 38%), Ara h 2 (n=13, 27%) and Ara h 6 (n=11, 23%).
Table 2: Results of mean papule diameter of skin prick (SPT) and skin prick-prick tests (SPPT) and mean values of serum-specific IgE levels (sIgE) (ImmunoCAP®) to the respective nuts.

Oral Food Challenges
A total of 17 OFCs were performed: 15 with non-culprit nuts that patients avoided despite absence of previous exposition and 2 to suspected nuts to clarify index reactions to a specific nut. Six (35%) OFCs were positive (4 with non-culprit nuts and 2 with the suspected nut). All these patients had mild cutaneous reactions (2 eczema exacerbation and 4 urticaria).
Anaphylaxis Predictors
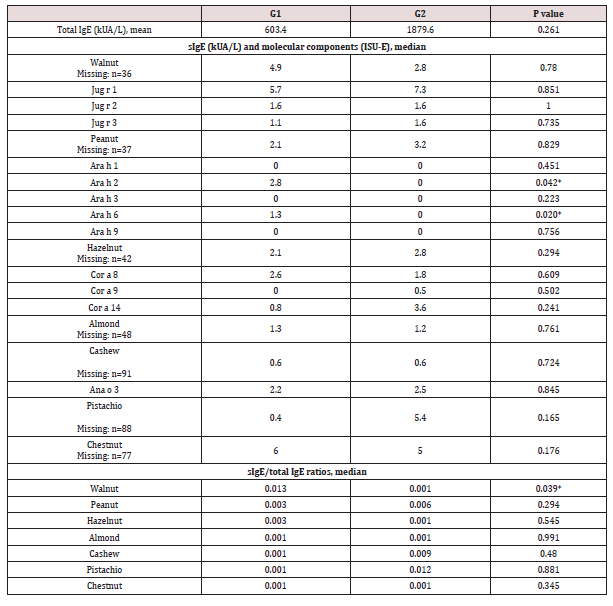
A significant association was found between anaphylaxis and allergy to chestnut (OR 5.023 [IC 95% 1.691-14.922], p 0.002) and cashew (OR 2.901 [IC 95% 1.184-7.107], p 0.018) (Figure 1). Chestnut anaphylaxis was present in 13 of the 19 chestnut allergic patients, 9/13 were male, all sensitized to other nuts, and only 3 reported chestnuts as the culprit of index reaction. On the other hand, cashew anaphylaxis was reported by 17/31 cashew allergic patients, 13/17 were male, 2 were monosensitized, 10 also presented pistachio allergy, and 8 reported cashews as the culprit of index reaction. Reaction severity was independent of the number of nut sensitizations (p 0.655). Considering SPPT, MPD was significantly higher in G1 for almond (6.5 vs 4mm, p 0.015), cashew (10 vs 5mm, p 0.049) and pistachio (8 vs 3.75mm, p 0.046) (Figure 2). sIgE values were not good predictors of reaction severity for any nut (p <0.05, Table 3). However, a significantly higher value of sIgE/total IgE ratio was found in G1 for walnut (0.0125 vs 0.0005, p 0.023). There was no significant association between symptom severity and serum eosinophils (682.9 vs 596.6/μL, p 0.261) or total IgE (603.4 vs 1879.6 kUA/L, p 0.068). As for the mcIgE, peanut Ara h2 and Ara h6 were identified in more patients from G1 vs G2, with higher median mcIgE values (2.8 vs 0 ISU-E, p 0.042; 1.3 vs 0 ISU-E, p 0.020) (Table 3). Jug r 1 was the most prevalent molecular component, although median values between groups did not differ significantly.
Figure 1: Differences between anaphylaxis (G1) vs non-anaphylaxis (G2) groups regarding culprit nuts *p < 0.05.
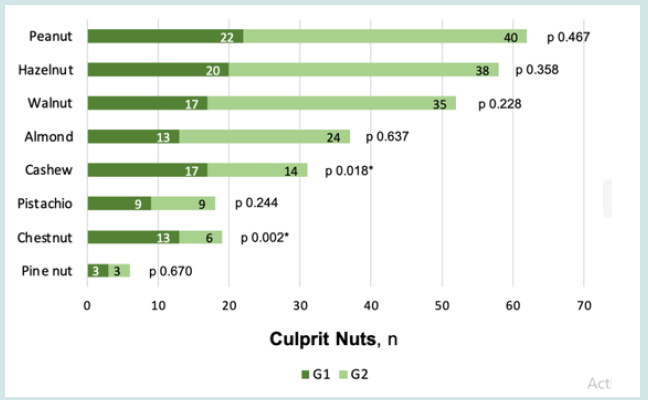
Figure 2: Mean papule diameter (MPD) of SPPT in both anaphylaxis (G1) vs non-anaphylaxis (G2) groups *p < 0.05.

Table 3: Comparison between groups (anaphylaxis G1 vs non-anaphylaxis G2) regarding Total IgE, sIgE, molecular components and sIgE/total IgE ratio *p < 0.05.
Discussion
In this retrospective Portuguese pediatric study that included 98 patients, the most frequent nuts involved were peanut (63%), hazelnut (59%) and walnut (53%). Nut reactions were reported earlier to peanuts than to other nuts (mean age at first reaction 4.6 years versus 6.7 years, p 0.02). We assume that it is a possible consequence of a recent increased consumption of nuts in our country, especially at an earlier age, predominantly of peanuts [17,18]. In Spain, Haroun-Díaz E et al. reported these same three nuts (hazelnut, peanut, and walnut) as the most frequent nuts eliciting allergy [19]. Allergy prevalence for each TN seems to vary in different regions of the world: hazelnut allergy is the most frequent in continental Europe; peanut, brazil nut, walnut and almond are the most commonly reported in the United Kingdom, and walnut and cashew allergies in the United States. These differences are representative of the variations in nuts consumption in each country, leading to different sensitization patterns [20,21]. Most patients had a history of atopy (n=86, 88%), including 44% with eczema and 31% with egg allergy.
Cetinkaya PG et al. reported a higher frequency of these atopic conditions in patients allergic to nuts, with 72% having atopic dermatitis and 50% egg allergy, probably related to a higher number of involved children [22]. The epithelial barrier dysfunction characteristic of atopic dermatitis is a confirmed risk factor for the development of allergic sensitization, food allergy and other allergic diseases. Many recent studies have demonstrated the connection between skin and digestive tract [23,24]. Damaged keratinocytes produce IL-33, that stimulates group 2 innate lymphoid cells (ILC2) in the small intestine. These in turn produce IL-4 and IL-13, which leads to the expansion of activated mast cells, resulting in an increment of intestinal permeability and consequent transmission of allergens that can trigger food allergy [25,26]. Allergic reactions to nuts may be severe on the first contact. We have observed in our population that about one third presented with anaphylaxis, which is in accordance with recent data of international cohorts. It should also be noted that more than half of our patients were polysensitized to several nuts, which is in line with recent literature.
Avoidance of all nuts has been the rule for many years in patients allergic to one nut, but the possible introduction of other nuts has recently been investigated in several studies [27]. In our study, 9 out of the 15 OFCs performed with non-culprit nuts to which patients were sensitized, but not exposed to before, were negative, favoring the above-mentioned idea of avoiding unnecessary nuts restrictions. Additionally, 31 (32%) were monosensitized after allergological investigation (16 to peanut, 7 to walnut, 4 to cashew, 2 to hazelnut and 2 to pine nut). IgE sensitization to pan-allergen LTP were the most prevalent in our population, differently from a Spanish cohort that found the 2S albumin family of the seed storage proteins as the most frequent. It is, however, in line with recently published Portuguese data, in which authors also found the same higher prevalence of sensitization to LTP [28]. In our pediatric cohort, anaphylaxis was more common in patients with chestnut and cashew allergies. Severe systemic reactions in patients sensitized to cashew have been frequently reported in Europe. A Portuguese study on TN anaphylaxis in preschool age children concluded that cashew was the major culprit, accounting for 11 of the 25 cases.
Data on chestnut allergy is sparce, especially considering prevalence and reaction’s severity. Our results could be explained by Portuguese eating habits, with chestnut being one of the most appreciated nuts. Allergological investigation proved to be useful in anaphylaxis risk prediction. MPD should be valued not only for diagnosis but also for anaphylaxis risk prediction. In our study, MPD was significantly higher for almond, cashew, and pistachio in patients with anaphylaxis to these TNs. Other authors reported the utility of ST in diagnosis prediction (MPD 8mm), but not in anaphylaxis risk prediction. sIgE did not differ significantly between groups. However, it should be taken into account that there was limited availability of whole extract sIgE for some of the nuts analyzed (namely cashew, pistachio, chestnut and pine nut) and that not all patients did sIgE measurements. In addition, as previously postulated, our study highlights the important adding value that sIgE/total IgE ratio could bring to clinical practice.
For example, sIgE measurement for walnuts was not a good predictor of anaphylaxis but, when integrated in sIgE/total IgE ratio, it reached statistical significance. Component-resolved diagnosis was used as a study complement, showing interest in risk stratification. In a recent Spanish study, Jug r 1, Ara h 2 and Ara h 6 were the most prevalent allergens, while Cor a 9, Cor a 14 and Ana o 3 were less prevalent. Similarly, in our study Jug r 1 was the most prevalent, although the difference between groups was not significant, Ara h 2 and Ara h 6 were also prevalent and associated with anaphylaxis, and Cor a 9, Cor a 14 and Ana o 3 were less represented. The present study has some limitations, such as the small sample of patients, which limits extrapolation of the results, and its retrospective design, that could weaken our findings. However, it is the first Portuguese study which extensively characterizes a pediatric population with TN and peanut allergy, highlighting the clinical utility of ST MPD and sIgE/total IgE ratio in anaphylaxis prediction, which has been sparsely reported, and so the authors believe that it could add significant value to clinical practice.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributions
LEC, RB, CV, FCD, CC: study design; LEC, RB, CV: data collection and analysis; LEC, RB: writing – original draft; CV, FCD, CC: writing – review & editing.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- Ibáñez-Sandin, Rodríguez Del Río P, Alvarado MI, García BE, Garriga-Baraut T, et al. (2022) Onset of Nut Allergy in a Pediatric Cohort: Clinical and Molecular Patterns in the AFRUSEN Study. J Investig Allergol Clin Immunol 32(4): 270-281.
- Weinberger T, Sicherer S (2018) Current perspectives on tree nut allergy: a review. J Asthma Allergy 11: 41-51.
- Eigenmann PA, Lack G, Mazon A, Nieto A, Haddad D, et al. (2017) Managing Nut Allergy: A Remaining Clinical Challenge. J Allergy Clin Immunol Pract 5(2): 296-300.
- McWilliam V, Koplin J, Lodge C, Tang M, Dharmage S, et al. (2015) The Prevalence of Tree Nut Allergy: A Systematic Review. Curr Allergy Asthma Rep 15(9): 54.
- Midun E, Radulovic S, Brough H, Caubet JC (2021) Recent advances in the management of nut allergy. World Allergy Organ J 14(1): 100491.
- Smeekens JM, Bagley K, Kulis M (2018) Tree nut allergies: allergen homology, cross- reactivity, and implications for therapy. Clin Exp Allergy 48(7): 762-772.
- Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA (2005) The natural history of tree nut allergy. J Allergy Clin Immunol 116(5): 1087-1093.
- Stiefel G, Anagnostou K, Boyle RJ, Brathwaite N, Ewan P, et al. (2017) BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin Exp Allergy 47(6): 719-739.
- King RM, Knibb RC, Hourihane JO (2009) Impact of peanut allergy on quality of life, stress, and anxiety in the family. Allergy 64: 461-468.
- Avery NJ, King RM, Knight S, Hourihane JO (2003) Assessment of quality of life in children with peanut allergy. Pediatr Allergy Immunol 14: 378-382.
- Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, et al. (2014) EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy 69(8): 1008-1025.
- Allergy & Autoimmune Disease, ImmunoCAP ISAC.
- Phadia Multiplexing Diagnostics GmbH. Phadia AB, Uppsala, Sweden; Wien, Austria: 2009.
- Muraro A, Worm M, Alviani C, Cardona V, Dunn Galvin A, et al. (2022) European Academy of Allergy, Clinical Immunology Food Allergy, Anaphylaxis Guidelines Group. EAACI guideline: Anaphylaxis (2021 update). Allergy 77(2): 357-377.
- INC International Nut & Dried Fruit Council (2023) Nuts & Dried fruits: Statistical yearbook 2017-2018.
- INE Instituto Nacional de Estatística (2023) Frutos secos, Portugal.
- Haroun-Díaz E, Azofra J, González-Mancebo E, de Las Heras M, Pastor-Vargas C, et al. (2017) Nut Allergy in Two Different Areas of Spain: Differences in Clinical and Molecular Pattern. Nutrients 9(8): 909.
- Brough HA, Caubet JC, Mazon A, Haddad D, Bergmann MM, et al. (2020) Defining challenge-proven coexistent nut and sesame seed allergy: A prospective multicenter European study. J Allergy Clin Immunol 145(4): 1231-1239.
- Marchisotto MJ, Harada L, Kamdar O, Smith BM, Waserman S, et al. (2017) Food Allergen Labeling and Purchasing Habits in the United States and Canada. J Allergy Clin Immunol Pract 5(2): 345-351.e2.
- Cetinkaya PG, Buyuktiryaki B, Soyer O, Sahiner UM, Sackesen C, et al. (2020) Phenotypical characterization of tree nuts and peanut allergies in east Mediterranean children. Allergol Immunopathol (Madr) 48(4): 316-322.
- O Neill CA, Monteleone G, McLaughlin JT, Paus R (2016) The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 38(11): 1167-1176.
- Levkovich T, Poutahidis T, Smillie C, Varian BJ, Ibrahim YM, et al. (2013) Probiotic bacteria induce a 'glow of health'. PLoS One 8(1): e53867.
- Tham EH, Rajakulendran M, Lee BW, Van Bever HPS (2020) Epicutaneous sensitization to food allergens in atopic dermatitis: What do we know? Pediatr Allergy Immunol 31(1): 7-18.
- Echeverría-Zudaire LÁ (2020) TREE NUTS ALLERGY: Knowledge, gaps, and practical implications. Allergol Immunopathol (Madr) 48(4): 313-315.
- Alves PB, Pereira HP, Alves MP, Roseta L, Tavares B, et al. (2022) Predictors of anaphylaxis to peanut and tree nuts in a Mediterranean population. Allergy Asthma Proc 43(6): 533-542.
- Matias J, Gaspar A, Sokolova A, Borrego LA, Piedade S, Pires G, et al. (2020) Tree nuts anaphylaxis in preschool age children. Eur Ann Allergy Clin Immunol 52(4): 182-186.
- Pascal M, Moreno C, Dávila I, Tabar AI, Bartra J, et al. (2021) Integration of in vitro allergy test results and ratio analysis for the diagnosis and treatment of allergic patients (INTEGRA). Clin Transl Allergy 11(7): e12052.
- Hoffman-Sommergruber K, Hilger C, Santos A, de las Vecillas L, Dramburg S, et al. (2022) Molecular Allergology User’s Guide 2.0, EAACI.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





