Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4722
Case Report(ISSN: 2637-4722) 
Tadalafil in Persistent Pulmonary Hypertension of the Newborn: Analysis of Eight Cases Volume 4 - Issue 5
Charbell Miguel Haddad Kury1*, Ana Carolina Pinto Mendes2, Elisa Carvalhal de Souza3, Thyara Boechat de Souza3, Rafaella Silva Souza3, Charles Bruno Soares3, Cinthia Guimarães Leandro2, and Bárbara Soares de Oliveira Souza3
- 1Coordinator of the neonatal intensive care unit (ICU) of the Saint John Baptist Hospital-municipality of Macaé, State of Rio de Janeiro, Brazil.
- 2Medical Student, Medicine School of Campos, municipality of Campos dos Goytacazes, State of Rio de Janeiro, Brazil.
- 3Medical Student, Federal University of Rio de Janeiro (UFRJ) – Macaé, State of Rio de Janeiro, Brazil. Study conducted in ICU of the Saint John Baptist Hospital – municipality of Macaé, State of Rio de Janeiro, Brazil.
Received: September 24,2024 Published: September 27,2024
Corresponding author: Charbell Miguel Haddad Kury, Coordinator of the neonatal intensive care unit (ICU) of the Saint John Baptist Hospital-municipality of Macaé, State of Rio de Janeiro, Brazil
DOI: 10.32474/PAPN.2024.04.000200
Abstract
Background: Therapeutic alternatives have been the subject of research for the treatment of persistent pulmonary hypertension of the newborn (PPHN). The use of tadalafil has shown to be a promising alternative to sildenafil. To analyze the evolution of eight newborns with PPHN admitted to an intensive care unit (ICU), which underwent therapeutic use of tadalafil in a daily dose of 1.5mg/kg/day every 24 hours.
Methods: Review of medical records to assess several variables of interest performed before and after intervention with tadalafil.
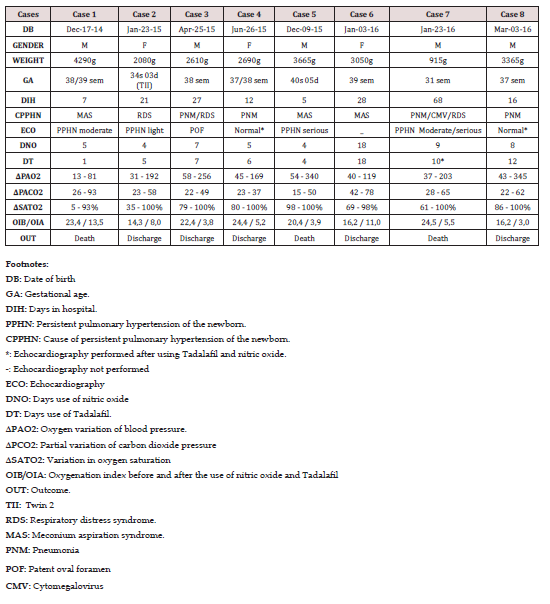
Results: The gestational age of neonates ranged from 31 weeks to 40 weeks and 5 days and the birth weight varied from 915 g to 4290 g. The minimum stay in the neonatal ICU was of five days and a maximum of 68 days. Regarding the main cause of PPHN, meconium aspiration syndrome (MAS) was accounted in three cases, followed by 2 cases for pneumonia and other causes such as respiratory distress syndrome (RDS); RDS associated with pneumonia and cytomegalovirus pneumomia (1 case of each). Three infants presented PPHN moderate to severe, according to the echocardiographic report. Use of nitric oxide (NO) varied from 4 to 18 days. Use tadalafil ranged from 1 to 18 days.
Conclusion: The use of tadalafil to control PPHN proved successful. However, further clinical studies to evaluate the safety and efficacy of this drug are needed.
Keywords: Persistent Fetal Circulation Syndrome; Tadalafil; Newborn
Introduction
The Persistent Pulmonary Hypertension of the Newborn (PPHN) is a condition characterized by inefficient transition from fetal pulmonary circulation to neonatal pulmonary circulation [1]. This is due to persistently elevated pulmonary vascular resistance, which does not suffer proper reduction after birth, thus requiring maintenance of right-left extrapulmonary shunts through the oval foramen and patent arterial channel [2]. The established framework prevents adequate pulmonary circulation and satisfactory blood oxygenation, causing arterial hypoxia [3]. It is estimated that the average incidence of PPHN is about 1.2-4.6 per 1000 live births with all-cause mortality of 20,6% in low and middle resources countries. For the other side, in the United States, the incidence of PPHN is estimated to be 0.18%, with one-year mortality of 7.6%. Preterm infants may also be affected, but the occurrence in this group is underestimated [4]. PPHN can be classified as primary or secondary to several neonatal cardiorespiratory diseases, such as respiratory distress syndrome (RDS), meconium aspiration syndrome (MAS), transient tachypnea of the newborn, congenital diaphragmatic hernia, congenital heart diseases, sepsis, pneumonia and birth asphyxia [5].
The treatment of this medical condition is based on routine use of nitric oxide and sildenafil [6]. However, alternative therapies have been subjects of research. In this work the use of tadalafil for controlling PPHN in 8 newborns admitted to a Neonatal Intensive Care Unit (NICU) was emphasized. This study aimed to demonstrate the benefit of the use of tadalafil in PPHN in eight newborns of the NCIU of the Saint John Baptist Hospital in the municipality of Macae, State of Rio de Janeiro, Brazil. These cases of PPHN have used tadalafil as part of the treatment, replacing sildenafil.
Materials and Methods
Local of the Study
This study was conducted in the neonatal intensive care unit (NICU) of the Saint John Baptist Hospital in the municipality of Macae, State of Rio de Janeiro, Brazil.
Study type
This was a case series study consisting of 8 babies presenting with PHHN and treated with tadalafil. An analysis of the medical records relating to cases of PPHN of the Neonatal Intensive Care Unit (NICU) was also performed.
Inclusion Criteria
a) Cases of PPHN in which the treatment using Tadalafil in a daily dose of 1.5mg/kg/day every 24 hours were introduced.
b) Babies presenting with PPHN which was attended in the NICU since December 2014 to March 2015.
Exclusion Criteria
a) Babies presenting any kind of severe malformations.
b) Babies presenting with PPHN out of the time scheduled.
Instrument and procedures for data collections
a) Select the cases to be studied.
b) Analysis of medical records.
c) Identify variables of the study: age, gender, weight, gestational age, days in hospital, Cause of persistent pulmonary hypertension of the newborn, Echocardiography, days of use of inhaled nitric oxide, days of use of tadalafil, Oxygen variation of blood pressure, Partial variation of carbon dioxide pressure, Variation in oxygen saturation, Oxygenation index before and after the use of nitric oxide and Tadalafil.
Statistical analysis
The cases and their variables were analyzed in the Excel program (Microsoft®).
Ethics
The study was sent to the Research Ethics Committee of the Faculdade de Medicina de Campos through the Plataforma Brasil system, and were approved on march 2017.
Results
Of the eight newborns evaluated in the study, five were males and three females. Neonates were of gestational age ranging between 31 weeks and 40 weeks and 5 days and the birth weight ranged between 915 g and 4290 g. The minimum stay in the neonatal ICU was of 5 days and a maximum of 68 days. The meconium aspiration syndrome was the major cause of PPHN included in the study, corresponding to three cases, followed by pneumonia, which included two patients. Other causes of persistent pulmonary hypertension in newborns analyzed in the study were: respiratory distress syndrome, respiratory distress syndrome associated with pneumonia and cytomegalovirus, and respiratory distress syndrome associated with pneumonia Seven of eight newborns underwent echocardiogram in the hospital. The results were normal in 2 newborns that underwent echocardiography after treatment with tadalafil and nitric oxide. Among other echocardiographic findings, patent oval foramen, mild PPHN, moderate PPHN, PPHN severe, and moderate / severe PPHN, were obtained. The use of nitric oxide ranged from 4 days to 18 days. 2 newborns used nitric oxide for 4 days (25%) and 2 used for 5 days (25%). The use of tadalafil ranged from 1 day to 18 days. Regarding the final clinical outcome, 5 newborns were discharged and 3 evolved to death (Table 1).
Discussion
This study was performed mainly in term infants (75%) of normal weight (75%). Pneumonia alone was the most common cause of PPHN (50%) or associated with respiratory distress syndrome or meconium aspiration syndrome. Several studies have associated some risk factors to the pathogenesis of PPHN just like: parenchymal changes pneumonia, sepsis, transient tachypnea of the newborn, meconium aspiration syndrome (MAS), chronic fetal distress and pulmonary hypoplasia resulting from congenital diaphragmatic hernia, being MAS the most common cause [6]. A case-control study also identified as risk factors for PPHN: black or Asian male individuals, and also maternal factors such as body mass index greater than 27 kg / m2, diabetes, asthma and born by cesarean section [8].
Considering the newborns studied, all underwent therapy with inhaled nitric oxide (iNO). Of these, four subjects remained with the iNO treatment for 4 to 5 days; 3 individuals, between 7 and 9 days and 1 subject for 18 days. It is known that nitric oxide is an endothelium-derived factor and by activating the guanylate cyclase, increases the levels of cyclic guanosine 3 ‘, 5’-monophosphate, causing vasodilation and also appears to increase the partial pressure of oxygen (PaO2). When inhaled, vasodilation pulmonary vasculature and presents minimal effect on the systemic circulation. Nitric oxide improves ventilation / perfusion by redirecting the blood of an area with a low rate to regions where gas exchange is normal [9-12]. The tadalafil used as drug therapy in the newborns assessed in this study is a medicament for oral administration and a phosphodiesterase-5 inhibitor approved for the treatment of pulmonary hypertension. In Brazil, tadalafil is approved for firstline treatment for acute pulmonary hypertension in adults [13]. In newborns, tadalafil has been used to treat PPHN as an adjuvant with inhaled nitric oxide (iNO). There are some reviews that describes the better efficacy, safety and cost in newborns, especially in comparison to sildenafil [4,14-17]. However, multicenter studies evaluating the use of tadalafil in neonates with PPHN are still needed.
Among those involved in the study, there was a significant drop in the oxygenation index (OI) and increased partial pressure of oxygen (PaO2) before and after the use of tadalafil associated with the use of inhaled nitric oxide. Reduction in the oxygenation index ranged from 32% to 83%. The increase in PaO2 ranged from 66% to 87%. The improvement in these parameters is consistent with some studies that evaluated the same parameters in neonates with pulmonary hypertension treated with sildenafil [18-20].
When assessing the outcome obtained, a positive result was observed in 5 individuals discharged in the period between 16 and 28 days. Of the 3 patients who presented an unfavorable outcome, one had PPHN pneumonia, cytomegalovirus (CMV) and respiratory distress syndrome. The other two deaths occurred at 5 and 7 days of hospitalization, both associated with meconium aspiration syndrome (MAS). The association between isolated cytomegalovirus infection and development of pulmonary hypertension in neonates is rare when other causes are excluded. It is suggested that the mechanism for the pathophysiological development PPHN is pneumonitis virus [21]. About 10 to 15% of infants with meconium aspiration syndrome developed PPHN with high mortality rate. The genesis seems to be pulmonary vasoconstriction due to inflammation [22].
The fact that there isn´t a control group to assess the efficacy and effectiveness of tadalafil compared to standard therapy recommended for newborns with PPHN was considered a flaw in the study, being necessary to compare the impact on improvement in oxygenation in time of hospitalization and outcome. The research involved a small sample. Thus, it is necessary to conduct multicenter studies involving more infants affected by PPHN.
Conclusion
The management of persistent pulmonary hypertension of the newborn (PPHN) remains challenging, contributing to the increase of morbidity and mortality in the neonatal period when improperly performed, in addition to requiring immediate invasive measures of Brazilian neonatal ICUs. The use of tadalafil as a main therapeutic drug for this clinical condition has shown promising in this study. However, we need more randomized controlled trials to evaluate the safety and efficacy of this intervention.
References
- Steinhorn RH (2016) Advances In Neonatal Pulmonary Hypertension. Neonatology 109(4): 334-344.
- Wei Zhang, Yue-E Wu, Xiao-Yan Yang, Jing Shi, John van den Anker, et al., (2020) Oral drugs used to treat persistent pulmonary hypertension of the newborn. Expert Review of Clinical Pharmacology 13(12): 1295-1308.
- Distefano G, Sciacca P (2015) Molecular physiopathogenetic mechanisms and development of new potential therapeutic strategies in persistent pulmonary hypertension of the newborn. Ital J Pediatr 8(1): 41-6.
- Nakwan N, Jain S, Kumar K (2020) An Asian multicenter retrospective study on persistent pulmonary hypertension of the newborn: incidence, etiology, diagnosis, treatment and outcome. J Matern Fetal Neonatal Med 33(12): 2032-2037.
- Hopper RK, Abman SH, Ivy DD (2016) Persistent Challenges in Pediatric Pulmonary Hypertension. Chest 150(1): 226-236.
- Nair J, Lakshminrusimha S (2014) Update on PPHN: mechanisms and treatment. Semin Perinatol 38(2): 78-91.
- Iacovidou N, Syggelou A, Fanos V, Xanthos T (2012) The Use of Sildenafil in The Treatment of Persistent Pulmonary Hypertension of The Newborn: A Review of The Literature. Curr Pharm Des 18(21): 3034-3045.
- Abman SH, Hansmann G, Archer SL, et al. (2015) Pediatric Pulmonary Hypertension: Guidelines from the American Heart Association and American Thoracic Society. 132(21): 2037-2099.
- Delaney C, Cornfield DN (2012) Risk Factors for Persistent Pulmonary Hypertension of The Newborn. Pulm Circ 2(1):15-20.
- Teixeira-mendonça C, Henriques-Coelho T (2013) Pathophysiology of pulmonary hypertension in newborns: therapeutic indications. Rev Port Cardiol 32(12): 1005-1012.
- Klinger JR, Abman SH, Gladwin MT (2013) Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med 188(6): 639-646.
- Steinhorn RH (2011) Therapeutic approaches using nitric oxide in infants and children. Free Radic Biol Med 51(5):1027-1034.
- (2013) Brazil Ministry of Health. Tadalafil for pulmonary hypertension. Brasília-DF.
- Ruopp NF, Cockrill BA (2022) Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review 12: 327(14):1379-1391.
- Lakshminrusimha S, Mathew B, Leach CL (2016) Pharmacologic strategies in neonatal pulmonary hypertension other than nitric oxide. Semin Perinatol 40(3):160-173.
- Takatsuki S, Calderbank M, Ivy DD (2012) Initial experience with tadalafil in pediatric pulmonary arterial hypertension. Pediatr Cardiol 33(5): 683-688.
- Shiva A, Shiran M, Rafati M (2016) Oral Tadalafil in Children with Pulmonary Arterial Hypertension. Drug Res (Stuttg) 66(1): 7-10.
- Gutiérrez Nicolás F, Viña Romero MM, González Carretero P, Merino Alonso J (2011) Sildenafilo oral para la hipertensión pulmonar en neonatos [Oral sildenafil for pulmonary hypertension in neonates]. Farm Hosp 35(3):157-158.
- Daga SR, Verma B, Valvi C (2008) Sildenafil for pulmonary hypertension in non-ventilated preterm babies. The Internet Journal of Pediatrics and Neonatology 8(1): 1-4.
- STEINHORN RH, Kinsella JP, Pierce C, et al. (2009) Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr 155(6):841-847.e1.
- Walter-Nicolet E, Leblanc M, Leruez-Ville M, Hubert P, Mitanchez D, et al., (2011) Congenital cytomegalovirus infection manifesting as neonatal persistent pulmonary hypertension: report of two cases. Pulm Med 293285.
- Lin HC, Wu SY, Wu JM, Yeh TF (2008) Meconium aspiration syndrome: experiences in Taiwan. J Perinatol 28 Suppl 3: S43-S48.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...