
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4722
Case Report(ISSN: 2637-4722) 
Non-Surgical Management of Life-Threatening Neonatal Pneumatoceles – Case Reports Volume 4 - Issue 5
Geza Vass*
- Geza Vass, Newborn Care Unit, John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust, Headley Way, Oxford, OX3 9DU, United Kingdom
Received: April 03,2024 Published: April 18,2024
Corresponding author: Nicola Sommer, University of Teacher Education, Institute for Educational Sciences, 5020 Salzburg, Akademiestraße 23-25, Austria
DOI: 10.32474/PAPN.2024.04.000198
Abstract
The management of large, symptomatic pulmonary pneumatoceles continue to be a challenge in neonates despite its improved incident since the introduction of surfactant, gentle ventilation and early extubation practices. There is no consensus for treating such cases. Large bullae can compress surrounding organs needing higher support and leading to a vicious circle. Surgery is high risk and only available in selected centers. We present three cases where 1) unilateral intubation of the contralateral side for 5 days or chest tube insertion by ultrasound guidance resulting in 2) draining or 3) rupturing the pneumatocele resulted in full resolution of the pneumatocele.
Introduction
Pulmonary pneumatoceles are gas-filled cystic changes in the lung parenchyma. They are part of neonatal air leak syndromes. Most are due to ventilator-associated lung injury or pneumonia1. Their incident has reduced from about 3.2% to 1.8% since surfactant therapy2, and likely even further improved since gentle ventilation and early extubation practices have been introduced. However, they continue to be present especially in the most extreme preterm. Most of pneumatoceles resolve spontaneously or improve in response to ventilation strategies such as use of HFO ventilation, low MAP or early extubation3, 4 Some, however, may progress to large bullae that compresses the surrounding organs leading to worsening respiratory and/or circulatory status needing higher ventilation settings and creating a vitious circle. Mortality rate in these cases is high and they often end up needing surgical lobectomy5. These procedures are high risk and require transfer to neonatal surgical centers. Here, we describe three cases with severe, life-threatening pneumatoceles unresponsive to ventilation strategies but resolved with other non-surgical interventions.
Case 1
A twin girl was born at 26+6 weeks` gestation with birth weight of 910 grams via vaginal delivery with background of oligohydramnios and chorioamnionitis. She required higher settings of mechanical ventilation in the first week of life. She was extubated on day 9 to high flow therapy. Her care was complicated with intraventricular haemorrhages and post-haemorrhagic ventricular dilatation which needed VP-shunt insertion. She was intubated for the surgery. On day 75, she was re-intubated for respiratory deterioration and was found to have a large right sided multi-cystic lesion in upper/ mid lob causing mediastinal shift and left lung collapse (Figure 1). Initial management with high frequency oscillation ventilation (HFOV) and low MAP strategy was not successful, she remained very unstable with high oxygen requirement and collapse of the contralateral lung. Selective intubation of left main bronchus first for 60 hours was unsuccessful, a second attempt for 5 days did not reach full collapse of the right side, but a third attempt with full collapse of the right side sustained for 120 hours (Figure 1) combined with low pressure HFOV resulted in complete resolution of pneumatocele followed by successful extubation on day 93. She was subsequently discharged home on day 121 on low flow oxygen.
Case 2
Extreme preterm twin girl was born at 25+5 weeks` gestation with birth weight of 750 grams via emergency caesarean section with background of chorioamnionitis. She had significant respiratory distress requiring mechanical ventilation and two surfactant doses. On day 12, her chest x-ray showed bilateral cystic lesions. The right cyst showed spontaneous resolution while the left one enlarged significantly causing high ventilator requirements. Her respiratory status was further complicated with pneumonia on 3 separate occasions. HFOV, steroids, and selective right sided intubation were unsuccessful; she remained ventilator-dependent. On day 49, a pigtail catheter was inserted into the pneumatocele using ultrasound guidance (Figure 2). The pneumatocele collapsed; with successful extubation on day 51. The chest tube was kept under suction similar to one used for pneumothorax until it stopped draining air. It was removed on day 65. The pneumatocele remained present on x-rays, but clinically insignificant until day 78 when it spontaneously disappeared. Baby was discharged home day 114 on low flow oxygen.
Figure 1: Series of chest X-rays depicting a large right sided pneumatocele causing full collapse of the contralateral lung and resolution with selective intubation
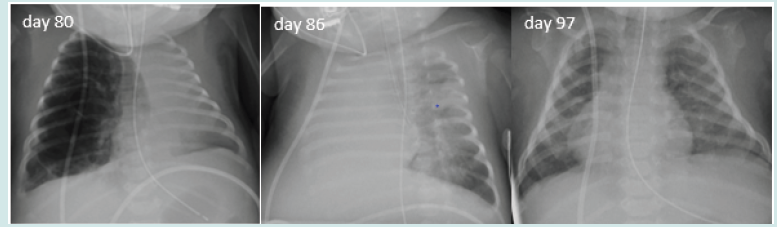
Figure 2: Series of chest x-rays (all done on day 49) showing left sided large bulla compressing the surrounding organs, its posterior position on lateral shoot-through and its resolution following a pigtail chest drain insertion (which was done using US guidance and from the back rather than from the side)
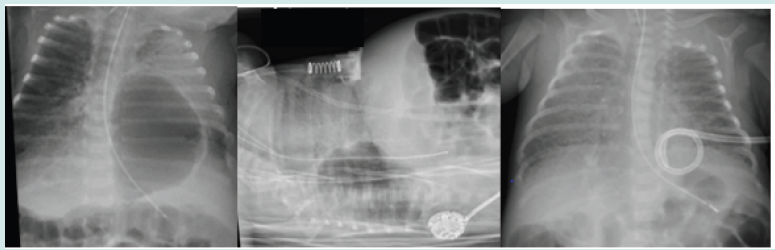
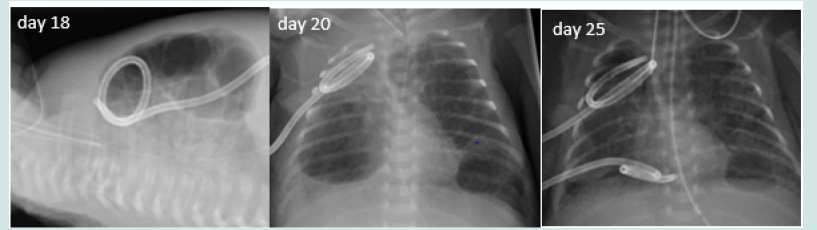
Figure 3: Series of chest x-rays showing the anterior position of the bilateral pneumatoceles, a previously inserted chest drain for a right sided pneumothorax, the single large bulla on the right side which was ruptured with the pigtail chest drain resulting in its resolution
Case 3
25+3 weeks` gestational age boy was born via normal delivery with birth weight of 755 grams. The mother had suspected chorioamnionitis and grew Candida from high vaginal swab. He was stabilized on high-flow oxygen therapy in delivery suit but was intubated soon after admission and had surfactant. Chest x-ray showed early PIE changes from day 4 onward. His early course was complicated with recurrent fungal septicaemias only resolving following multiple central line replacements. From day 12 onward, his x-rays showed evolving multiple bilateral pneumatoceles. He developed a right sided pneumothorax on day 17 needing chest drain insertion. He remained critically unwell (despite resolution of his pneumothorax) with high oxygen requirement (100%).
Multiple ventilation strategies, muscle relaxation and sedation made no improvement. Lateral x-ray confirmed the bullae to be anterior and he only tolerated to be nursed in prone position; the slightest handling or rotation resulted in profound and prolonged hypoxic events. Pulmonary hypertension was ruled out by echocardiography. In view of this, a drain insertion was attempted on the right side which had the largest single bulla. The drain ruptured the bulla and created a pneumothorax which was drained within one day using the previously inserted (and still in situ) chest drain. Following this, the pneumatocele on the right side gradually resolved. The contralateral bullae improved as well likely due to improved and thus reduced ventilatory settings. He was successfully extubation on day 41. His care was complicated with milk curd obstruction needing laparotomy, stoma formation and later reversal. He is currently still an inpatient and progressing well with no respiratory support.
Discussion
Majority of pneumatoceles (more than 85%) are asymptomatic and resolve spontaneously. However, if they become symptomatic, they often cause significant challenges and carry increased risk of mortality. Surgical interventions (e.g. lobectomy) are available but only in highly specialized centers and they do carry high risk morbidity and mortality as well as long term implicationsv. In nonsurgical centres other alternate interventions performed by the neonatal team can be attempted.
We presented three cases with severe, life-threatening pneumatoceles. All likely developed as a result of the combination of extreme prematurity, mechanical ventilation and inflammatory processes (chorioamnionitis, infections) as described in the literature. Because none of the lesions failed to resolve spontaneously but continued to cause severe cardio-respiratory compromise, we employed 2 different conservative approaches: 1) selective intubation and 2) ultrasound (US) guided percutaneous drainage using pigtail catheter. The underlying lung pathology leading to the development of a pneumatocele often poses a challenge with the selective intubation/unilateral ventilation as the risk for developing a pneumothorax on the unaffected side is high. Finding a sufficiently low pressure that keeps the unaffected side open can be challenging (likely the cause of the failure of the unilateral ventilation in our Case 2). It is difficult to estimate how long the selective intubation should be instituted for a full resolution. Joseph LJ at al6.
suggests in their comprehensive review of the available case reports that at least 48 hours is needed. Our experience (with other cases) is similar meaning 48-72 hours of unilateral intubation usually sufficient to resolve air leak syndromes (refractory pneumothorax, pneumatocele). However, are some cases like in our first case, a longer period (in this case ~120 hours) may be needed while making sure that the collapse of the contralateral side is complete. Selective unilateral intubation can be difficult. It is technically more difficult to do on the left side (technique described well by Chalak et al7.). Transient hypoxia (with or without bradycardia) is a well described complication. It is due to the ongoing perfusion of the collapsed lung leading to a ventilation/perfusion mismatch. After a few minutes, a self-regulated vascular response reduces the shunt and improves the oxygen saturation. Migration of the endotracheal tube is common. All these were observed with our case 1. For our case 2, a selective intubation was not tolerated despite being successful technically. As the pneumatocele continued to increase in size despite low pressure HFO ventilation leading to respiratory deterioration and a vitious circle, a more radical approach was warranted.
A lateral x-ray indicated a posterior location of the pneumatocele. Owing to critically sick nature of the baby`s illness, CT/fluoroscopic guided drainage was not feasible. A bedside US guided drain insertion was employed. This resulted an immediate collapse of the pneumatocele and rapid improvement of the respiratory status (successful extubation 3 days later), but the chest drain continued to drain air for further 2 weeks indicating a sustained connection with the main airways despite collapse. Although the pneumatocele has not fully resolved following the drain removal, it remained small enough to spontaneously disappear. While US guidance was helpful, the anterior-posterior and lateral x-rays were sufficient to identify the right insertion point for the chest drain. In retrospect, it is unlikely that a longer selective intubation would have been successful in case 2. Our case 3 had bilateral large bullae and was critically unwell, therefore neither selective intubation nor surgical lobectomy was feasible. A drainage of the largest bulla on the right hand side (similar to case 2) was attempted, but in this case it resulted in rupturing the bulla. It has nevertheless improved the clinical status and led gradual improvement of the contralateral bullae as well. It is speculated that the improvement of the contralateral bullae was due to the overall improved respiratory status and reduced ventilator settings.
There are very few cases reported in the literature to be managed successfully with the aforementioned techniques. No consensus approach exists for treating such complex pneumatoceles. While surgery may be felt to be the only solution, it does carry a high risk itself and only available in selected centres (meaning that the patient may need to have a high-risk transfer first). Selective bronchus intubation of contralateral side, drainage or rupture of the large bulla by chest tube insertion are less risky, but feasible and potentially successful alternate treatment options.
References
- Rocha G (2020) Pulmonary pneumatoceles in neonates Pediatr Pulmonol 55(10): 2532-2541.
- Hussain N, Noce T, Sharma P (2010) Pneumatoceles in preterm infants – incidence and outcome in the post‐surfactant era. J Perinatol 30(5): 330‐336.
- de Bie HM, van Toledo-Eppinga L, Verbeke JI (2002) Neonatal pneumatocele as a complication of nasal continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed May 86(3): F202-3.
- Wellington AC, Spigland NA, Worgall S (2015) Decreasing mean airway pressure as a management strategy for tension pneumatocoeles. Arch Dis Child Fetal Neonatal Ed Mar100(2): F101.
- Sacks GD, Chung K, Jamil K (2014) Surgical salvage of acquired lung lesions in extremely premature infants. Pediatr Surg Int. 30(5): 573‐576.
- Joseph LJ, Bromiker R, Toker O (2011) Unilateral Lung Intubation for Pulmonary Air Leak Syndrome in Neonates: A Case Series and a Review of the Literature. Am J Perinatol 28(2): 151-156.
- Chalak LF, Kaiser JR, Arrington RW (2007) Resolution of pulmonary interstitial emphysema following selective left main stem intubation in a premature newborn: an old procedure revisited. Paediatr Anaesth 17(2): 183-186.
- Muniraman H, Chintala S, Richardson R (2020) Successful ultrasound guided percutaneous drainage of pneumatocele in an extremely preterm infant. Radiol Case Rep 16(3): 607-611.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




