Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4722
Case Report(ISSN: 2637-4722) 
Legg -Calvé-Perthes Disease in a Pediatric Patient: Case Report Volume 3 - Issue 3
Lina Marcela Ortiz Roncallo1*, Angie Katerine Rodríguez Paredes2, Eyleen Elena Borja Alemán3, Ana María Valencia Castaño4, Adriana Paola Beltrán Miranda5, Olga Vanessa Manrique Arismendy6, Diana Marcela Bolaños Lamilla7, Estefanía Cordero Lugo8 and Ricardo Antonio Rendón Muñoz9
- 1General Physician, Universidad de la Sabana, Bogotá, Colombia
- 2General Physician, Universidad Cooperativa de Colombia
- 3General Physician, Universidad del Sinú, Montería, Colombia
- 4General Physician, Fundación Universitaria San Martín, Medellín-Colombia
- 5General Physician, Universidad del Sinú, Montería, Colombia
- 6Fourth-year medical student, Universidad Pedagógica y Tecnológica de Colombia
- 7General Physician, Universidad Libre seccional Cali, Colombia
- 8General Physician, Universidad Libre de Barranquilla, Colombia
- 9General Physician, Universidad Remingtón de Medellín
Received: September 24, 2021; Published:October 08, 2021
Corresponding author:Lina Marcela Ortiz Roncallo, General Physician, Universidad de la Sabana, Bogotá, Colombia
DOI: 10.32474/PAPN.2021.03.000163
Abstract
Legg-Calvé-Perthes disease is a rare disorder in children in which the rounded femoral head stops receiving blood supply. As a result, the femoral head collapses. The body will absorb the dead bone cells and replace them with new bone cells. Eventually, the new bone cells will return the head of the femur to shape. Legg-Calvé-Perthes disease causes pain and stiffness in the hip joint for a time. It is important to mention that this disease usually occurs in children between 2 and 12 years of age and in most cases, only one of the hips is affected.
Keywords:Legg-Calves-Perthes; Bone Disorder; Pediatric Legg-Calves-Perthes
Introduction
ELCP is a hip disorder of unknown origin. The disease is caused by a temporary interruption of blood flow in the ossification nucleus of the proximal epiphysis of the femur. After this blood interruption, osteonecrosis, and a loss of the physiological shape of the femoral head appear [1]. ELCP is an important pathology, since it affects the growing skeleton and alters the development of the femoral head, leaving sequelae that can be very serious. This evolution depends on the degree of vascular involvement, the alteration at the anatomical level and the type of treatment applied to the patient [2]. Other authors define ELCP as: “Idiopathic avascular necrosis of the epiphysis of the femoral head and its associated complications for the developing child” [3].
This disorder goes through four phases that affect the head of the femur. The phases include
a) Phase 1: initial. Lack of blood supply to the femoral head. There is swelling, stiffness, and pain in the hip joints. Death of bone tissue in some portions of the bone. On x-rays, the head of the femur appears less rounded. This phase can last from several months to a year
b) Phase 2: fragmentation. The body will remove the dead bone cells and replace them with new, healthier bone cells. The femoral head begins to assume the rounded shape again. There is still irritation and pain in the joints. This phase can last from one to three years.
c) Phase 3: ossification. The head of the femur continues to take its rounded shape with new bone tissue. This phase lasts between one and three years.
d) Phase 4: healing. Normal bone cells replace the new bone cells. This last phase can take a few years to complete the healing process [4, 5].
The cause of Legg-Calvé-Perthes disease is not known with certainty. The chances of it occurring in a boy are four times higher than in a girl and it rarely occurs in African American patients. Generally, the child complains of pain in the hip, which intensifies when she does some physical activity. Pain may also occur in the thigh or knee areas [6]. In most cases, the child limps, and reports that rest relieves pain. Over time, he may notice muscle loss in his upper leg and hips. The symptoms of this disease may resemble those of other associated clean hip conditions or pathologies [7].
Presentation of the Clinical Case
A 7-year-old male patient in poor general condition, who was admitted to the emergency department debuting with a clinical picture of 72 hours of evolution, consisting of pain in the left hip, with radiation to the lower left limb and hip, intensity 9/10 according to an analogous pain scale, which intensifies with standing and is associated with lameness exacerbated by trauma 3 days ago, with a radiographic finding compatible with avascular necrosis of the femoral head associated with joint effusion. On physical examination, patient with vital signs in normal parameters, hemodynamically stable, without alterations in the nervous system, ischoric pupils normo reactive to light, anicteric stairs, eyes without secretions, moist oral mucosa without lesions, bilateral otoscope without alterations, mobile neck, No masses or palpable lymphadenopathy, symmetrical expandable chest, without drawing its sacks, on pulmonary auscultation a universal vesicular murmur, well ventilated lungs, without added sounds, rhythmic heart sounds, well timbred, nor murmurs, nor aggregates. Abdomen without scars, water noises present, soft, not painful on superficial or deep palpation, without signs of peritoneal irritation, without masses or palpable megaly, unexplored external genitalia, symmetrical extremities, pain on palpation, at hip level left, with antalgic gait, inadequate support of the lower left limb, capillary filling <2 seconds, hydrated anicteric skin, no edema, no obvious lesions. Aware, alert patient with no apparent systolic or motor deficit without neurological targeting, Glasgow 15/15. The patient is left under observation, with an initial diagnostic impression of transient synovitis, with a venous plug and a normal diet. Naproxen 125/5 mil # 1 syrup is administered, give 6.3cc every 12 hours for pain. Hemogram, CRP, ESR, X-ray of the left femoral head is requested and an orthopedic evaluation with the results is requested.
a) 07/14/2021: semi-qualitative PCR: 1 MG / dl, Erythrosedimentation: 20 mm / h.
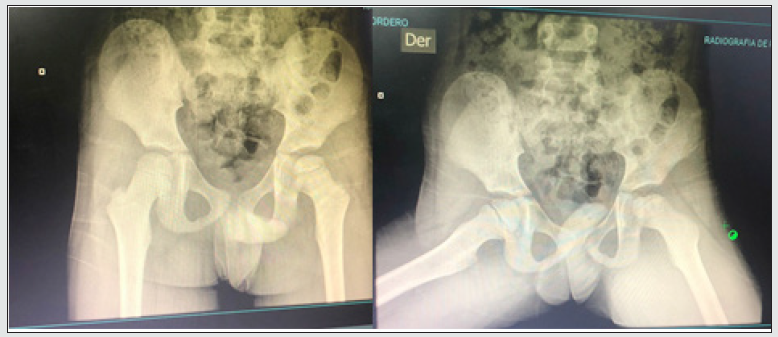
b) 06/15/2021: X-ray of the left femoral head showing irregularity and sclerosis of the secondary ossification nucleus of the femoral head, with necrosis of the same and an increase in the femoroacetabular joint space that gives us a diagnostic impression of avascular necrosis in the femoral head left (Leggcalves- perthes). Nuclear resonance imaging of lower limb joints (pelvis, knee, foot and / or standing neck) is requested.
c) 07/15/2021: Nuclear MRI of lower limb joints (pelvis, knee, foot and / or standing neck) with report of juvenile osteochondroosis, left femoral head (Legg-calves-perthes disease) with increased fluid in the joint capsule in the left hip.
d) 07/17/2021: Orthopedic assessment: a 7-year-old male pediatric patient with ex of the left femoral head and simple MRI of the left hip with a report of visualized bone structures without traces of fractures, lithic or expansive lesions. Distortion of the left hip structure is shown, with developing disorders of the femoral head, a tendency to flat coca, sequelae inherent to Legg-calves-perthes disease. No soft tissue grades. Conclusion: sequelae of Legg-calves-perthes disease in the left hip. Patient who decides to hospitalize to define surgical conduct, with venous plug, normal diet, dipyrone ampoule: 1 gr. Place 630mg IV now. At rest and with activity restrictions, a bed with rails above and vital signs control is requested.
Discussion
Legg-Calvé-Perthes disease (PCL) was initially described in 1909. as an affectation of the juvenile hip (8,9). Currently, PCL disease is a term reserved for idiopathic avascular necrosis of the juvenile hip, which can be associated with different pathophysiological mechanisms on its etology, which generates delays in the diagnostic suspicion as well as in the treatment [10, 11]. Different risk factors have been associated, in which the aim is to bring the clinician closer to an earlier and more timely diagnosis, however, none have managed to be the cause of the disease [12]. Among the many possible etiologies of the disease, the following are mentioned: sickle cell disease, systemic inflammatory diseases, chemotherapy, radiation, prolonged use of systemic steroids, trauma, vascular abnormalities, and increased mechanical load on the hip [13, 14]. Patients with PCL disease present an absence of blood supply at the level of the femoral head, which generates aseptic bone necrosis and, consequently, a crushing of the femoral head occurs. As time progresses, resorption and remodeling are generated at the level of the femoral head. PCL disease usually occurs in the pediatric population in an age range from 2 to 12 years. Which mostly affects the male gender more with a ratio of 4: 1 compared to the female sex and approximately 10-15% of cases the femoral head is affected bilaterally [15]. It should be emphasized that it is a disease in which its insidious onset and the establishment of clinical manifestations may not be florid, where mostly the evolution corresponds to its pathophysiology, with this it has been possible to define a relationship between the history according to its radiological stage natural and its clinic. Four phases of the disease have been described according to the radiological changes that the patient presents at the level of the femoral head: initial phase or necrosis phase, fragmentation phase, re-ossification phase and final phase or healing phase [16, 17].
During the natural course of the disease, the child remains afebrile since what occurs is an aseptic osteonecrosis at the level of the femoral head without any other inflammatory data initially, at this stage the first radiological signs of the necrosis phase can be found. are the displacement of the femoral head laterally with respect to the acetabulum and the flattening of the femoral head, as it evolves, greater displacement can be observed, including fragmentation of the femoral head, which would mark the second radiological phase, the third phase is delimited from the moment in which there is no further advance of the initial disease, the remodeling of the femoral head and ends with complete healing, this being a phase where it is necessarily important to define the therapeutic intervention [18]. Although the etiology is unknown, attempts have been made to involve different factors as responsible for this disease. Among them are the presence of a possible synovitis, coagulation disorders, thrombophilia, short stature and delayed bone age, repeated trauma, taking corticosteroids, low socioeconomic status, etc. However, none of them have been proven [19]. It is estimated that the disease is suffered by between 1.0 and 2.5 of every 10,000 children. It predominates between 4 and 5 times more in boys than in girls, but in these the prognosis is worse [20]. The disease manifests itself between 3 and 8 years. The patients are usually thin, very active and smaller than their contemporaries. It is bilateral in 10-15% of cases. Bilaterality is never synchronous, so when one hip is in the reosification phase, it begins on the other side. There is a higher incidence in Japanese and Eskimos and it is rare in blacks, Australian aborigines, Native Americans, and Central Europe [21]. Radiographic imaging is the most common, widely used, easy to achieve, inexpensive, and well-known method of diagnosing and evaluating ELCP. They should be practiced if this or another condition of the hips or pelvis is suspected; they rule out or help their classification and prognosis; they also provide the basis for deciding the treatment.
Anteroposterior (ap) and lateral images should be taken in Lauenstein position (frog); These images are the basis for placing the disease in some stage of the different classifications that are used [22]. The X-rays show the signs of early diagnosis of Catterall [23] Lateral displacement of the femoral head, subchondral fracture line, increased epiphyseal density, smaller size of the epiphyseal nucleus in the affected hip compared to the healthy one. Data that if the radiographs have been carried out very early, they will not be seen and we will only find characteristics of synovitis, which for Waldenström [24] only sees a widening of the joint space, which lasts from one to three weeks and when there is a homogeneous increase in the opacity of the femoral head is already in the period of aseptic or avascular necrosis that lasts from a few months to a year. In the regeneration or fragmentation stage, areas of rarefaction with the appearance of rounded fragments are distinguished, since there is vascular fibrous tissue between the bone or immature bone without calcification, the femoral neck widens and there may be extrusion of the femoral head. In the residual stage, the rarefaction areas disappear and there is replacement by normal bone, so the femoral head recovers its sphericity or is flattened [25]. (For Tachdjian [26] the first radiographic signs are:
a) Nucleus of ossification of femoral head small compared to contralateral
b) Subchondral fracture line in the femoral head.
c) Increased radiopacity of the femoral head [27].
After establishing the diagnosis, it is important to remember that the goal of treatment is to preserve the rounded shape of the head of the femur and prevent it from deforming during the disorder. Treatment options depend on the degree of pain and stiffness of the hip. In addition to the changes shown by radiographs over time and the portion of the femoral head that has collapsed [28].
The primary goal in treating Legg-Calvé-Perthes disease is to obtain an early diagnosis of the disorder to allow as much time as possible for the head of the femur to return to its rounded shape. Other goals of treatment include controlling pain, maintaining hip mobility, and preventing the deformity from getting worse. The two most important factors in determining the outcome are the child’s age and how affected the head of the femur is due to the disorder; the more severe the case, the more likely the child will experience restriction of hip movement, differences in leg length and hip problems in adulthood [29]. Since we do not know the etiology of the disease, its prevention is not possible Any patient suspected of suffering from Perthes disease should be referred to the orthopedic surgeon for a complete evaluation of the case, in mild cases, the follow-up can be done from primary care [30]. The type of treatment depends on the severity of the disease. In mild cases, and as the natural evolution is toward cure, treatment consists of bed rest, use of crutches, restriction of sports activities, and rehabilitation. For the treatment of pain and inflammation, analgesics and rest are recommended. In patients with severe pain and great loss of hip movement, admission to a hospital may be necessary to better control rest and medication or to place immobilizations to relax the muscles. Once the pain has disappeared, physical therapy or hydrotherapy can be started [31]. In more advanced stages, the main objective of treatment is to try that the shape of the femur head adapts during its development, which ends at 8 years of age, as best as possible to the shape of the cup (containment theory), since this would act as a mold of the femoral head in formation and thus achieve a congruent articulation. In this way, it is possible to minimize the development of hip deformities that could lead to the development of osteoarthritis in adulthood. This can be achieved with the use of orthoses, tenotomies of the adductor muscles and iliopsoas, and in severe cases with surgery performing femoral and pelvic osteotomies [32]. The disadvantage of performing osteotomies is that a second intervention must be performed to remove the material that was used to fix the femoral head within the cup. In cases of significant contracture of the adductor muscles, treatment with botulinum toxin combined with intensive physical therapy can be performed. The use of orthoses and the restriction of movement can cause psychological and social problems in children compared to children of their age, so if it is decided to carry out this type of treatment, children and their families may need the attention of a psychologist [33].
The best strategy for deciding which treatment to apply is to consider the age at which the symptoms appeared combined with the degree of involvement of the femoral head at that time. Applying this principle, we can establish the following treatment guidelines.
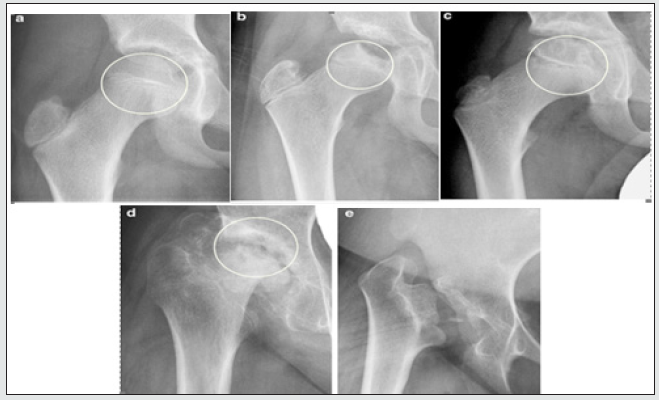
a) Age less than 6 years: In this age group the findings correspond to Stulberg’s classes I and II (Figure 1) and good results are obtained with conservative treatments. If in this age group we find cases compatible with Stulberg’s class III, surgical treatment does not contribute anything with respect to conservative treatment, so the latter is preferred. In the cases belonging to groups, I and II of Catterall. (Figure 2) treatment should be conservative. If they belong to groups III and IV, it will be assessed by magnetic resonance whether there is lateral extrusion of the femoral head, if there is not, the treatment will be conservative; but if there is extrusion or other signs of risk, the treatment would have to be surgical [34]. If the findings correspond to groups A and B of the Herring lateral pillar classification (Figure 3), the treatment will be conservative. But if they correspond to group B / C or C, the treatment will be surgical [35].
b) Age 6 and 8 years: The type of treatment to be carried out in this age group is less clear. Some authors affirm that there are no differences between being conservative or surgical. If the findings correspond to groups A and B of the Herring lateral pillar classification, the treatment will be conservative. But if they correspond to group B / C or C, the treatment will be surgical [37].
c) Age older than 8 years: In the cases that correspond to groups B / C and C of the Herring lateral pillar classification, and for certain authors, also those that correspond to group B, surgical treatment is recommended. In this age group, with findings compatible with Catterall’s groups I and II, surgical treatment is applicable, but it is only effective in 41% of cases [37]. In cases corresponding to Stulberg’s classes III and IV, the treatment will be surgical. There are studies that show that from 8 years of age surgical treatment does not offer advantages over conservative treatment since it is no longer possible to prevent or reverse the deformity of the femoral head. Regarding the «hinged» hip, as it is a deformed hip with compromised mobility, the treatment must be surgical [38].
Conclusion
It is evident that the ELCP continues to be conceived as a pathology in constant investigation, especially in the fact of trying to definitively determine its etiology, to favor a better management of it, in general. Once a diagnosis is defined, advanced complementary tests can be carried out, which not only allow the staging of the disease but also prevent complications and know its prognosis. The clinician in knowledge of this will be able to identify patients with PCL disease early to make a referral of the case to a specialized level in a timely manner where the patient can be offered the best and most timely therapeutic approach. Among the difficulties that this pathology means, the greatest is the clinical ignorance of it, which makes its diagnosis late, in addition to the difficult access in some populations to health services and the high costs of complementary tests.
References
- Kliegman R Nelson (2012) Treatise on Pediatrics 19th Spain: Elservier Sounders pp. 2438-2440.
- Mancilla E, Sánchez I, Beltramino D, García A (2013) Meneghello Pediatrics 6th USA Pan American pp. 2592-2595.
- Vázquez J, Díaz J (2008) Diagnosis and Treatment in pediatrics. Mexico Modern Manual pp. 751-754.
- Bentley G (2014) European Surgical Orthopedics and Traumatology 1st United Kingdom Springer pp. 4443-4468.
- Ramachandran M, Reed DW (2016) Legg-Calvé-Perthes Disease of the Hip. Orthop Trauma 30(6): 1-10.
- Laville JM (2010) Legg-Perthes-Calvé EMC-Apar Locomot 43(3): 1-10.
- Salcedo Montejo M, González Morán G, Albiñana Cilveti J (2011) Legg-Calvé-Perthes disease. Rev Esp Cir Ortop Traumatol 55 (4): 312-22.
- Marcdante K, Kliegman R (2012) Essential of Pediatrics 7th Philadelphia (USA): Elservier Sounders pp.674-675.
- Vargas Carvajal IX, Martínez Ballesteros ÓF (2012) Legg-Calvé-Perthes disease. Updated review Semergen 38(3): 167-74.
- Arbaiza P, Zambrano J (2007) Legg-calvé-perthes disease bibliographic review. Rev Medicine 13(2): 140-147.
- Shah H (2014) Perthes disease: evaluation and management. Orthop Clin North Am 45(1): 87-97.
- Miranda L, Bas T, Martí V (2005) Perthes disease Basic concepts. An Pediatr Contin 3 (5): 317-321.
- Bertol P (2004) Legg-Calvé-Perthes Disease. Rev Bras Ortop 39(51): 543-554.
- Atanda Jr A, Shah SA, O Brien K (2011) Osteochondrosis: common causes of pain in growing bones. Am Fam Physician 83: 285-291.
- Kim HK (2010) Legg-Calvé-Perthes disease. J Am Acad Orthop Surg 18: 676-686.
- Munoz, Calvo MT, Hidalgo Vicario MI, Clemente Pollán J (2008) Extra-hospital pediatrics. Clinical Foundations for Primary Care 4th Madrid Ergon pp. 859-881.
- atterall A (1971) The natural history of Perthes disease. J Bone Joint Surg 53B: p.37.
- Catterall A, Pringle J, Byers PD (1982) A review of the morphology of Perthes’ disease. J Bone Joint Surg Br 64: 269-275.
- Salter RB (1966) Experimental and clinical aspect of Perthes disease. J Bone Joint Surg 48B: pp.393.
- Salter RB, Rang M, Blackstone IW, McArthur RC, Weighill FJ, Gygi AC (1977) Stulberg SD: Perthes’ disease the scientific basis of methods of management and indications. J Bone Joint Surg 59B: pp.127.
- Morrissy R, Weinstein SL (2001) Lovell and winter’s Pediatric Orthopaedics 5th Philadelphia, Lippincott Williams & Wilkins 2: 957-991.
- Canale S, Beaty S (1992) Treatise of Pediatric Orthopedics, London, Mosby-Year Book pp. 747-760.
- Waldenström H (1923) On coxa plana. Acta Chir Scand 55: pp.577.
- Waldenström H (1934) The first stage of coxa plana. Acta Orthop Scand 4: p.1.
- Waldenström H (1938) The first stage of coxa plana. J Bone JointSurg 20: pp.559.
- Tachdjian M (1990) Pediatric Orthopedics 2nd ed, Philadelphia. WB Saunders Company pp.933-988.
- Conway JJ, Weiss SC (1983) Maldonado V Scintigraphic patter in Legg-Calvé-Perthes disease. Radiology 102: pp.167.
- Tsao AK, Dias LS, Conway JJ, Straka P (1997) The prognostic value and significance of serial bone scintigraphy in Legg-Calvé-Perthes disease. J Pediatr Orthop 17: 230-239.
- Futami T, Kasahara Y, Suzuki S (1991) Utrasonography in transient synovitis and early Perthes’ disease. J Bone Joint Surg 73: 635-639.
- Eckerwall G, Hochbergs P, Wingstrand H, Egund N (1994) Sonography and intracapsular pressure in Perthes’ disease: 39 children examined 2-36 month alter onset. Acta Orthop Scand 65: 575-580.
- Kaniklides C, Lonnerholm T, Moberg A, Sahlstedt B (1995) Legg-Calvé-Perthes disease: comparison of conventional radiography, MG imaging, bone scintigraphy and arthrography. Acta Radiol 36: 434.-439.
- Pouletaut P, Claude L, Winzenrieth R (2005) Automated analysis of RM image of hip: geometrical evaluation of the Legg-Calvé-Perthes’ disease. Med Eng Phys 27: 415-424.
- Hoffinger SA, Henderson RC, Renner JB (1993) Magnetic resonance evaluation of metaphyseal changes in Legg-Calvé-Perthes disease. J Pediatr Orthop 13: 602-606.
- Uno A, Hattori T, Noritake K, Suda H (1995) Legg-Calvé-Perthes disease in the evolutionary period: comparison of magnetic resonanceimaging with bone scintigraphy. J Pediatr Orthop 13: 362-367.
- Gent E, Antapur P, Fairhurst J (2006) Perthes’ disease in the very young child. J Pediatr Orthop B 15: 16-22.
- Sales de Gausy J, Briot J, Swider P (2009) Coxa magna quantification using MRI in Legg-Calvé-Perthes disease. Clin Biomech 24(1): 43-46.
- Sundt H (1949) Malum coxae Calvé-Legg-Perthes. Acta Chir Scand 148: p.1.
- Stulberg SD, Cooperman DR, Wallensten R (1981) The natural historyof Legg-Calvé-Perthes’ disease. J Bone Joint Surg Am 63: pp.1095.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...