Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4722
Case Report(ISSN: 2637-4722) 
Kenny Caffey Syndrome, Clinical and Genetic Features in Children in North Israel Volume 2 - Issue 5
Wael Nasser3*, Haia Nasser1, Bayan H4, Amir Bar1, Jerdev Michael2, Ehsan N4, Boshra N3 and Susan Nasser3
- 1Department of Pediatrics, Baruch Padeh Poriya Medical Center, Israel
- 2Department of Radiology, Baruch Padeh Poriya Medical Center, Israel
- 3Nephrology & Hypertension Division, Baruch-Padeh Poriya Medical Center, Isreal
- 4Department of Pediatrics, Ziv Medical Center, Israel
Received: June 15, 2020 Published: August 04, 2020
Corresponding author: Wael Nasser, Nephrology & Hypertension Division, Baruch Padeh Poriya Medical Center, Azrieli Faculty of medicine, Israel.
DOI: 10.32474/PAPN.2020.02.000148
Abstract
Kenny-Caffey syndrome (KCS) type 1 is a rare hereditary skeletal disorder. KCS reported almost exclusively in middle eastern populations. It is characterized by severe growth retardation-short stature, dysmorphic features, episodic hypocalcemia, hypoparathyroidism, seizures, and medullary stenosis of long bones with thickened cortices. We present here three cases with the characteristic symptoms of growth retardation, dysmorphic features, and hypoparathyroidism. After a full investigation, Kenny- Caffery Syndrome was diagnosed.
Keywords:Kenny-Caffey, Hypocalcemia, Hypoparathyroidism, Sanjad-Sakati syndrome
Introduction
Kenny-Caffey syndrome is a rare genetic condition causing skeletal abnormalities. The disease affects males and females in equal proportion. Inheritance may be autosomal dominant or autosomal recessive. Individuals with the condition have a shortened stature and thickened long bones. Electrolytic disturbances are prevalent, like hypocalcemia. It is a rare primary bone dysplasia syndrome characterized by cortical thickening and medullary stenosis of the long bones, growth retardation with short stature, delayed anterior fontanelle closure, facial dysmorphism micrognathia, and microphthalmia, congenital hypoparathyroidism leads to hypocalcemia. An additional manifestation is an optic atrophy, tortuous retinal vessels, dental caries, enamel defects, and, occasionally, hypoplastic nails and neonatal liver disease. [1,2] with more severe growth retardation, intellectual disability, microcephaly, and recurrent bacterial infections being observed in the latter. Kenny-Caffey syndrome type 1 (KCS 1) is a rare autosomal recessive skeletal disorder. This disease has been found almost exclusively in the Middle East. A few data available about its prevalence is in Saudi Arabia and is estimated between 1:40,000 and 1:100,000 live births [3]. In 1995 in Israel, six Bedouin families in the country were first described as having the syndrome, and they showed endocrine involvement, low stature, and secondary hypoparathyroidism; There are cases with participation of the central nervous system and the immune system and also accompanied by developmental delays.
Case 1
A two-week-old baby boy was administered to our department due to restlessness and vomiting. He was born to consanguineous parents from bedouin Origin. Patient history revealed that he was borne at term by normal vaginal delivery in a private hospital and cried immediately at birth. His birth weight was 2.5kg and length were 34.5cm. In the father’s family, a number of children died at an early age due to unknown reason. On physical examination: a very small baby with facial dysmorphism. Same weight as birth weight 2.5kg, no pathological finding on auscultation of the heart and lungs. non-pitting edema in the legs and hands. The umbilical area appeared to be contaminated. Investigation to exclude sepsis was done and showed, hypocalcemia 5.2mg%, phosphorus 8.2mg%, magnesium 1.4mg%, albumin 3.5g%, the rest of the electrolytes were normal. X-ray images showed suspicion of thymus absence, considering that it is Di-George Syndrome, neck US was done and showed thymus. The baby began receiving large doses of calcium intravenous + 1 alpha vitamin 3D and magnesium sulfate. Calcium values reached the normal levels after about 3 weeks of intra-venous treatment with 750-1000mg/kg. + 0.5 micrograms of vitamin D. The baby stabilized at calcium values between 7.5- 8.5mg%. No weight gain was observed, despite proper intake of food (suction + maternity supplement). At the age of 6 months the child was readmitted and hospitalized due to cyanosis and apnea. On his admission the infant’s weight 3850g. Laboratory tests showed calcium levels 5.5g%, with phosphorus levels 9.64mg% and magnesium 1.6mg%. Complete blood count electrolytes and venous gases was normal. Intravenous calcium was applied: this time the calcium increased rapidly. follow up once a week was done in pediatric nephrology clinic. At age of 8 months, the patient’s laboratory tests were normal due to daily oral calcium therapy: 2500-10,000mg/day + Vitamin 3D α1 0.5μcg/d + phosphor adsorbents. In respect of the patient’s family history, Genetic consult suggested a possible hereditary disorder with autosomal recessive type of inheritance.
Case 2
A 6-month year old baby girl was administered to our hospital due to failure to thrive (FTT), she was thriving poorly in spite of her good appetite. She was the third child to consanguineous bedouin parents who had 2 children who died in childhood due to ventricular arrhythmias, due to injury to the CASQ 2 gene, and two more children passed away for an unknown reason. whereas the other siblings were normal. The baby was born after a fullterm pregnancy with no considerable maternal health issues. Her weight was 2.323kg at week 36. On examination, she was found to be severely growth retarded with normal mentality, her weight was 4.375kg, height 60cm, and head circumference 38cm (all far below third percentile), dysmorphic facies: (microcephaly, depressed nasal bridge, beaked nose tip, thin lips, micrognathia, and prominent forehead, thin legs, very small fingers in both hands and feet. Systemic examination including the cardiovascular system was within normal limits. Neurological examination was done with no significant findings. Investigations revealed that hemogram, liver, renal function, and urine analysis were normal. Laboratory tests showed low Calcium levels 5.4mg/dL, Phosphorus: 8.8mg/dL, Magnesium: 1.4mg%. Albumin: 3.9g%. PTH: 2.67pg/ml. The baby needed very large doses of calcium 1g/kg intravenously for about 2 weeks + vitamin D. Calcium values stabilized around 8.5mg%. The baby visited the clinic once a week for follow up, at age 23 months her weight was 4.775kg, Height 49cm (8 standard deviations below average). Brain Ultrasound showed central and peripheral atrophy. She has considerable developmental delays: recently started to roll over, not sitting, still head lag can be seen. Developmentally she is suitable for 4 months.
Case 3
A 2-year-old baby boy from northern Syria was hospitalized in Safed Ziv Hospital through the army. Due to general weakness and seizures it was hard to take medical history. Physical examination showed dysmorphic, microcephalic, micrognathia, short statures, psychomotor delay suitable for eight months ( 5 percent standard deviation below 3% percent for weight and height).Laboratories revealed normal blood count, normal kidney and liver function, normal lipid profile, he had low calcium and high phosphorus levels (calcium 5.5mg/dL, phosphorus 8.5mg/dL). normal albumin, no alkalosis or acidosis was demonstrated in gases. Repeated tests for PTH and vitamin D levels were very low (PTH levels 3.5PG / ML, vitamin D 5 NNG/ML). Normal thyroid function, normal IGF1 level, and normal cortisol levels. Chest x-ray with no significant findings, Renal ultrasound without nephrocalcinosis with normal renal function. The child hospitalized for three weeks and was treated. Genetic Counseling gave the diagnosis of Kenny Caffey syndrome matched the mutation (Hypoparathyroidism dysmorphic retardation).
Discussion
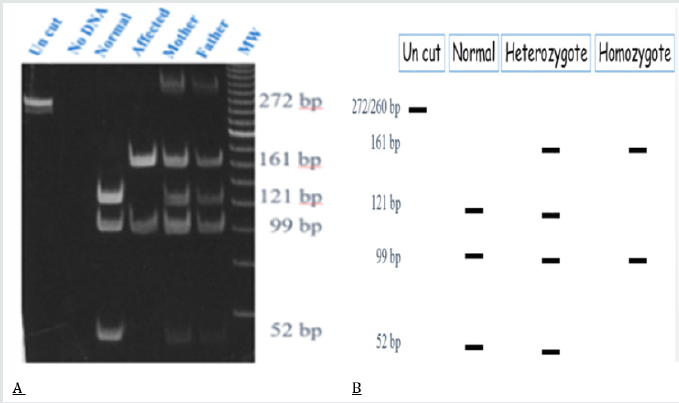
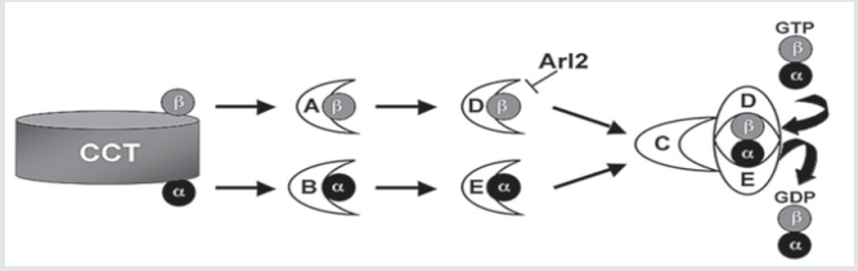
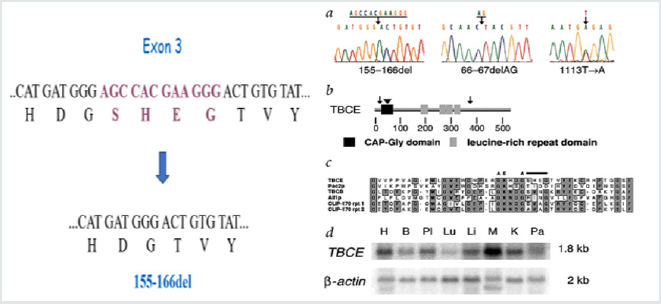
Kenny- Caffey syndrome is a rare hereditary skeletal disorder, first reported by Kenny and Linarelli in 1966. Caffey described its radiological features in 1967 [4,5]. The autosomal recessive form of Kenny-Caffey syndrome. Has a defect in the TBCE gene. The TBCE gene consists of 17 axons, the mutation causing the disease is 155-166 deletion; in Israel the mutation was noticed mostly in Bedouin origin in southern Israel (Figure 1 & 2). this mutation was found in other patients of Bedouin origin in middle eastern countries, recently the same mutation was found in patients belonging to the Bedouin tribe from northern villages. The clinical characteristics of the patients include, growth failure congenital hypoparathyroidism, mental retardation, facial dysmorphism, and severe growth failure. Patients with Kenny-Caffey type 1 also have osteosclerosis and immunodeficiency. Sanjad-Sakati syndrome has been reported almost exclusively in arabs families. Both disorders map to 1q42-q43 and are caused by mutations in the TBCE gene (tubulin-specific chaperone E), which encodes a protein involved in tubulin folding (Figure 3). This finding suggests the two disorders are likely allelic or constitute different reports of the same condition. Yet another similar phenotype, Kenny-Caffey syndrome type 2, is an autosomal dominant disorder due to heterozygous mutations in the FAM111A gene (family with sequence similarity 111, member A) and differs from the type 1 syndrome in its absence of mental retardation [6,7].
Figure 1: The 155-166del mutation causes the disease in patients with Kenny-caffey syndrome in the northern region of Israel.
Differential Diagnosis of Neonatal Hypocalcaemia
Calcium is actively transferred from the mother to the fetus by a calcium pump regulated by PTHrP. The result is that in newborn calcium values are higher than in the mother, Calcium values: 10- 11 mg/dL. Definition of hypocalcaemia depends on age and birth weight: In neonates> 1500gram hypocalcaemia when total Calcium is < from 8mg%, and ionic calcium <4.4mg%. In premature infants with weight <1500gram, hypocalcemia: with total calcium <7mg% and ionic calcium <4mg%. In neonates of diabetic mothers, it is common to see post-natal hypocalcemia (10-50%) often associated with hyperphosphatemia. About 1/3 of the calcium levels are low in the first two days. Usually due to hypoalbuminemia, a disturbed response to PTH, an increase in calcitonin, an increased urinary excretion following increased sodium excretion, and birth asphyxia can cause hypocalcaemia. In addition, it can be noticed in infants with IUGR, Probably due to poor calcium transfer through the placenta. These neonates are usually asymptomatic. Late hypocalcaemia after 48-96 hours: secondary to hypoparathyroidism, Di George, hypomagnesemia, high phosphorus consumption (cow’s milk). Differential diagnosis of neonatal hypocalcaemia should include, Digeorge syndrome, about 80-90% of these children have a chromosome 22q11 microdeletion. And a diagnosis is made with the help of genetics tests. CATCH 22: cardiac defects, abnormal faces, thymic hypoplasia, hypocalcemia, hypoparathyroidism.
Hypoparathyroidism
A group of clinical and biochemical syndromes in all lacking PTH, hypocalcemia, and hyperphosphatemia such as transient hypoparathyroidism, relatively common in neonatal. congenital hypoparathyroidism although it is rare, Congenital hypoparathyroidism may appear alone or as part of various syndromes and chromosomal alterations. Sporadic cases, X -linked recessive have been described Xq26-27. And Kenny-caffey Syndrome, this disease has been observed almost exclusively in the Middle East. In the classic form, endocrine involvement: short stature secondary to hypoparathyroidism, with skeletal involvement significant thickening of the cortex of long bone with medullary stenosis, normal intelligence. There are cases with involvement of the central nervous system and the immune system and also accompany developmental delays. Another differential diagnosis is Sanjad-Sakati syndrome (SSS), an autosomal recessive disorder. The syndrome first described in 1988 with dysmorphic facial, severe hypocalcaemia, accompanying tetany, with or without seizures, begins at early childhood. It is thought that KCS 1 and (SSS) are caused by a mutation in the tubulin specific chaperone E gene (TBCE) gene on chromosome 1q42-43. The TBCE gene targets one of a number of chaperones needed for proper folding of alphatubulin subunits and formation of alpha-beta-tubulin heterodimers. This mutation has been found in other Bedouin patients in Middle Eastern countries. The findings of medullary stenosis and cortical thickening were not mentioned in SSS making the diagnosis of KCS 1 more probable.
References
- Kenny-Caffey Syndrome (2012) National Organization for Rare Disorders.
- Kenny-Caffey syndrome type 2 (2017) Online Mendelian Inheritance in Man (OMIM).
- Naguib KK, Gouda SA, Elshafey A, Mohammed F, Bastaki L, et al. (2009) Sanjad-Sakati syndrome/Kenny-Caffey syndrome type 1: A study of 21 cases in Kuwait. East Mediterr Health J 15: 345–352.
- Kenny FM, Linarelli L (1966) Dwarfism and cortical thickening of tubular bones. Transient hypocalcemia in a mother and son. Am J Dis Child 111: 201-207.
- Caffey J (1967) Congenital stenosis of medullary spaces in tubular bones and calvaria in two proportionate dwarfs-Mother and son; Coupled with transitory hypocalcemic tetany. Am J Roentgenol Radium Ther Nucl Med 100: 1-11.
- Sabry MA, Zaki M, Shaltout A (1998) Genotypic/phenotypic heterogeneity of Kenny-Caffey syndrome. J Med Genet 35:1054-1055.
- Kenny-Caffey syndrome type 2 (2017) Online Mendelian Inheritance in Man (OMIM).

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...