Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4722
Research Article(ISSN: 2637-4722) 
Aquatic Exercise Intervention Is Effective for Spasticity Inhibition in Children with Cerebral Palsy: A Clinical Controlled Study Volume 3 - Issue 1
Bolarinwa Isaac Akinola*
- Department of Physiotherapy, College of Medicine, University of Lagos, Pmb 12003, Idi-Araba, Lagos, Nigeria
Received: January 28, 2021 Published: February 15, 2021
Corresponding author: Bolarinwa Isaac Akinola, Department of Physiotherapy, College of Medicine, University of Lagos, Pmb 12003, Idi-Araba, Lagos, Nigeria.
DOI: 10.32474/PAPN.2021.03.000156
Abstract
Introduction: Spasticity has been implicated as a major hindrance to motor development and overall functional performance in children with cerebral palsy (CP). Recent evidence have suggested aquatic therapy as an alternative means for inhibiting spasticity in children with CP. However, these previous studies have provided contrasting results, thereby creating some dearth of evidence in the use of aquatic therapy to manage spasticity in children with CP.
Objective: To investigate the effectiveness of aquatic exercise intervention in inhibiting spasticity in children with CP.
Materials and methods: Thirty children aged 1-12 years participated in this study. They were randomised into 2 groups (experimental and control). The experimental groups received manual passive stretching on affected muscles of both upper and lower extremities followed by weight bearing exercises in water (temperature 28-32ºC) while the control group received same exercises on land for 10 weeks. Degree of spasticity on treated group of muscles was assessed using Modified Ashworth Scale (MAS). Mann-Whitney-U test was used to compare the change in degree of spasticity between both groups. The level of significance was set at p<0.05.
Results: The experimental group showed significant reduction in spasticity of all the tested muscle groups while the control group showed no significant improvement in spasticity of wrist flexors and knee flexors. No significant difference was observed for change in spasticity between both groups.
Conclusion: Aquatic exercise intervention is effective for inhibiting muscle spasticity in children with cerebral palsy.
Keywords: Aquatic Exercise; Intervention; Spasticity; Cerebral Palsy.
Introduction
Spasticity is a widespread problem among children with cerebral palsy (CP) as it affects function and can lead to musculoskeletal complications [1]. It occurs as a result of pathologically increase in muscle tone and hyperactive reflexes mediated by a loss of upper motor neuron inhibitory control [2]. Spasticity is considered an important neural contributor to muscle hypertonia in children with cerebral palsy [3].It adversely affects muscles and joints of the extremities, causing abnormal movements, and it is especially harmful in growing children[4]. Reduction of spasticity and improvement of motor control is a prerequisite for functional performance in children with cerebral palsy [5,6]. With reduction in spasticity, improvement in gross motor function and independent performance of ADLs are expedited which in turn reduces the burden cerebral palsy poses on the children and their caregivers [5,6,7] .The management of spasticity in children with CP is often complex and poses a great challenge to healthcare professionals. Effective treatment requires a multidisciplinary approach involving pediatricians, physiotherapists, occupational therapists, orthotists and surgeons. Previous studies have suggested various treatment approaches and modalities to manage spasticity associated with spastic CP. These include the use of oral neuropharmacological agents, injectable materials such as botulinum – a toxin or surgical treatment, use of orthotic devices, massage techniques, strengthening exercises to the antagonist musculature, use of electrical stimulation and the application of cryotherapy or ice therapy [8,9]. Aquatic intervention is one of the most popular supplementary treatments for children with neuromotor impairments, particularly CP [10]. Several studies have reported aquatic intervention as a veritable tool in the rehabilitation of children with CP [11-13]. The intervention may provide safe and beneficial alternative low-impact exercise for children with disabilities [14]. This study was aimed at investigating the effectiveness of aquatic exercise intervention in inhibiting spasticity in children with spastic cerebral palsy.
Methods
Thirty children undergoing rehabilitation were recruited for this study from a center for developmental challenges in Lagos, Nigeria. Their age range was from 1 to 12 years and were diagnosed with the spastic form of cerebral palsy. Those with other neurodevelopmental conditions were excluded from the study.
Ethical Approval and Informed Consent
Ethical approval for this study was obtained from the Health Research and Ethics Committee of the Lagos University Teaching Hospital, Idi-Araba, Lagos, Nigeria (Ref. No: ADM/DCST/HREC/ APP/1525). Parents of the participating children gave their informed consent.
Procedure
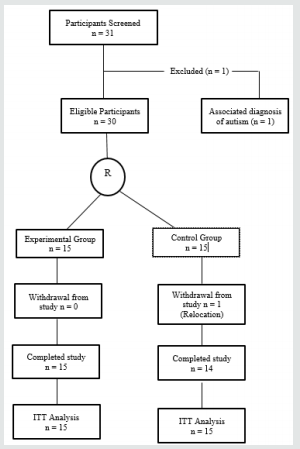
A detailed history and thorough physical examination was initially carried out on each participant. Those who met the criteria for the study were then randomly assigned into experimental and control groups. The flow chart of the study is presented in Figure 1.
Assessment Protocol
Functional Level Assessment: This was assessed using the Gross Motor Function Classification System- Expanded and Revised according to standard [15].
Degree of Spasticity Assessment: This was done using the Modified Ashworth Scale (MAS). Both upper limbs (shoulder adductors, elbow flexors and wrist flexors) and lower limbs (hip adductors, knee flexors and ankle plantar flexors) were assessed for spasticity bilaterally (left and right) according to the pattern presented by the participants. Each participant was placed in a relaxed supine position and the starting position of testing each muscle group was a maximal placement of the joint in a position of their primary function and then maximally moved to a lenghtened position opposite to their primary movement. Participants were then scored based on the amount of resistance to passive movement as described in MAS. To accommodate the score of “1+” modification for numeric analysis, grade ‘‘1+’’ was recorded as 1.5[16].
Intervention
The participants in both experimental and control groups had a total of 20 treatment sessions consecutively for 10 weeks at the rate of two sessions per week. It was ensured that participants in both groups were not on any form of anti-spastic medication throughout the study.
Those in the experimental group received exercise training in water of temperature ranging between 28-32 ºC for 10 weeks with the exercised parts fully immersed in water. The exercise protocol consisted of 3 categories of exercises:
Exercise 1 (Manual passive stretching): Each joint involving the spastic group of muscles was passively stretched and held in this in fully lengthened position of the muscle groups for up to 60 seconds follow by relaxation of 30 seconds. This procedure was repeated five times for each part
Exercise 2: Weight bearing one or both upper extremities in sitting with or without support for 60 seconds and repeated 5 times.
Exercise 3: Weight bearing on both lower extremities in standing with or without support for 60 seconds and repeated 5 times.
Control group
Participants in the control group received same exercise intervention as those in the experimental group except that the intervention was carried out on land.
Post-intervention Assessments
Participants in both groups were re-assessed after 4 weeks, after 8 weeks and after 10 weeks of intervention for changes in spasticity using the Modified Ashworth Scale (MAS). All assessments were carried out by blinded assessors.
Data Analysis
Data was analyzed using the Statistical Package for Social Sciences version 21.0 version. Participants in both groups were compared for age using the Independent t-test. Friedman test was used to compare post intervention changes in degree of spasticity within each group across the duration of treatment while Mann- Whitney U test was used to compare the change in spasticity between both groups. All statistical tests were performed at the 0.05 level of significance (p<0.05).
Results
Physical Characteristics
The mean age of all participants was 5.20+2.43 years and no significant difference in age was found between both groups of participants at baseline Table 1,2. Most of the participants were in level IV of the GMFCS. Quadriplegic spastic type of CP predominated among all the participants as shown in Figure 2.
Table 1: Comparison of Age and Mobility Level between both Groups at Baseline.
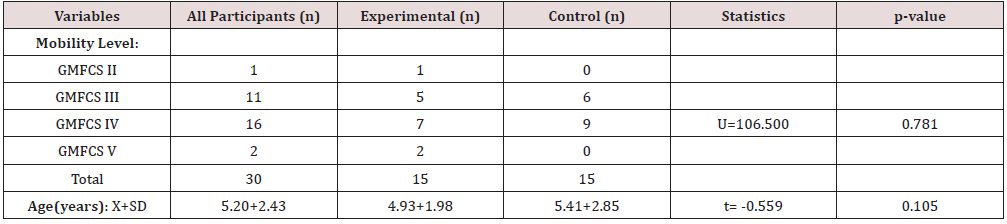
Key: X+SD: Mean + Standard deviation.
Aquatic Intervention and Spasticity
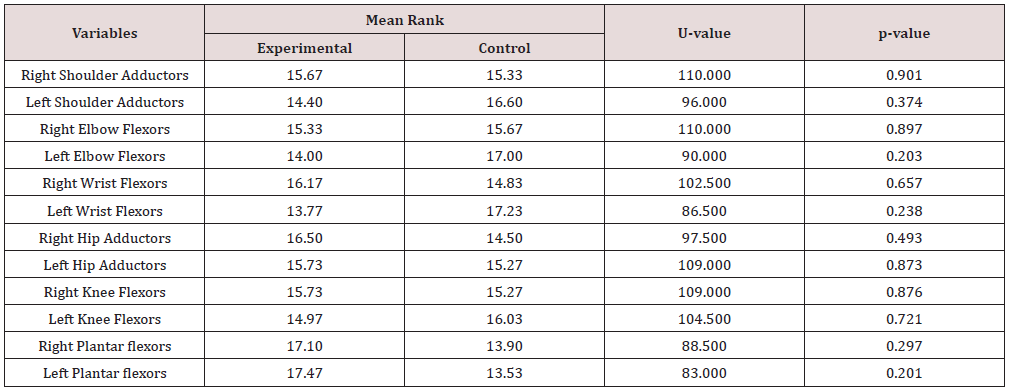
Significant change in spasticity was observed on all tested group of muscles of participants in the experimental group following aquatic exercise intervention Table 3.
Land-based Exercises and Spasticity
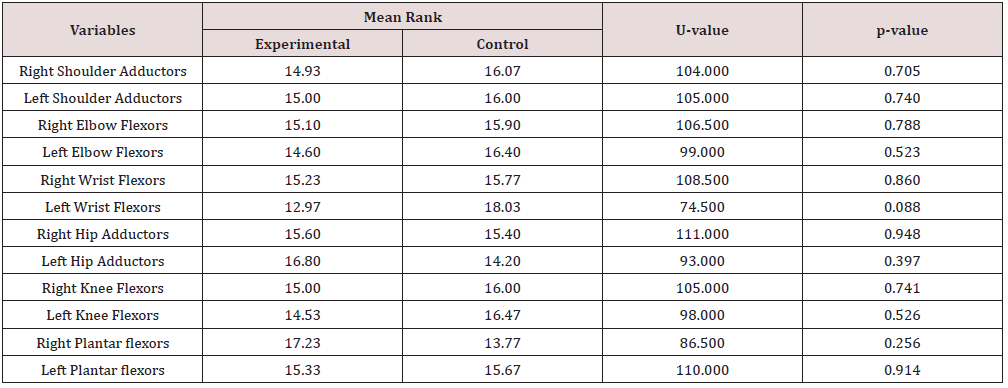
Significant change in spasticity was also observed on tested group of muscles among participants in the control group following land-based exercise intervention, except for right and left wrist flexors; and right and left knee flexors as shown in Table 4.
Table 3: Comparison of Mean Rank Changes in Spasticity in Experimental group of Participants across the duration of Intervention.
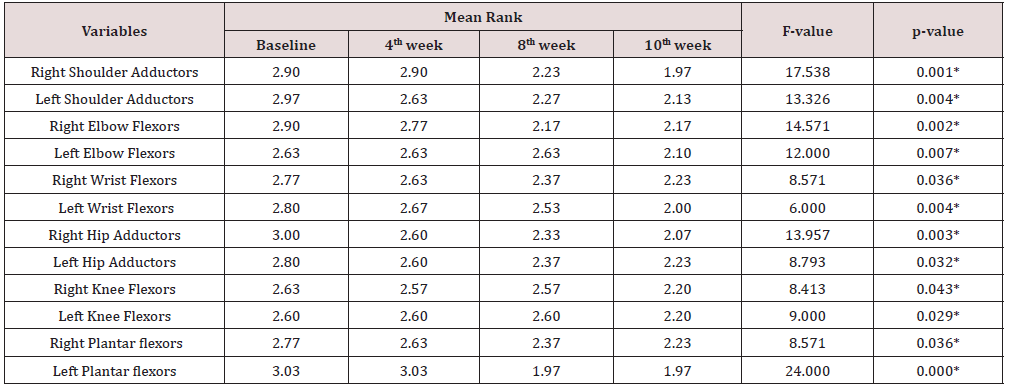
*Significant at p<0.05
Table 4: Comparison of Mean Rank Changes in Spasticity in Control group of Participants across the duration of Intervention.
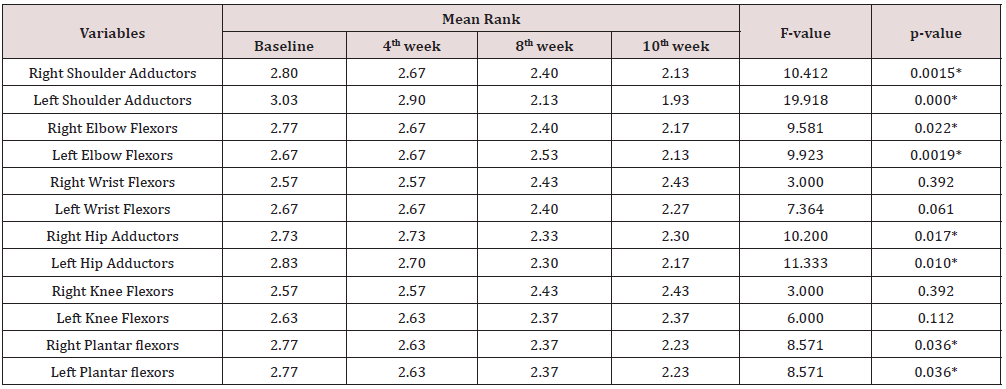
*Significant at p<0.05.
Comparison of Spasticity Change between Experimental and Control Groups
Despite the gross improvement in spasticity of participants in experimental group compared to the control group which showed no improvement for few groups of muscles, no significant difference was found in the spasticity change between both groups as represented in Table 5.
Discussion
Quadriplegic type of spastic CP was more predominant in this study. This is consistent with reports from earlier studies in Nigeria [17-19]. However, reports from studies in other parts of the world showed that there were more diplegic type of spastic CP [11,20][21- 23]. Geographical differences may be considered for this variation.
In this present study, statistically significant improvement was observed in both intervention groups for spasticity of shoulder adductors, elbow flexors, hip adductors and ankle plantar flexors. This finding shows that therapeutic exercises such as manual passive stretching and weight bearing exercises which are used by physiotherapists in the management of spasticity in children with cerebral palsy are effective as documented in previous studies [21,24,25,26]. The report of statistically significant improvement in spasticity of hip adductors and knee flexors following 10 weeks of aquatic intervention in this study has been supported by a study by Chrysagis et al in 2009 where there was further significant improvement in the passive range of motion of the hip and knee joints [11]. However, the aquatic training adopted for their own study was different from the one used in this present study. This improvement in spasticity is attributable to the therapeutic benefit of buoyancy property of water which can help to relax spastic muscles by providing antigravity positioning. This study revealed that landbased exercise intervention improved the spasticity of shoulder adductors, elbow flexors, hip adductors and ankle plantar-flexors as observed among participants in the control group. This finding was corroborated by a systematic review on the effectiveness of passive stretching on spasticity in children with cerebral palsy [24]. They found out that 4 of the 6 reviewed studies reported significant reduction in spasticity including that of shoulder adductors, elbow flexors, hip adductors and ankle plantar flexors following passive stretching while the remaining 2 studies did not consider spasticity in their outcome variables. This improvement in spasticity may be attributed to fatigability of stretch receptors as a result of stretch on the muscle spindles which induces inhibition on the gamma-motor neurons and allowed more lengthening of muscle fibres thereby counteracting the effect of spasticity.
There was no significant improvement observed for spasticity of the wrist flexors and knee flexors in this present study after 10 weeks of land-based exercises. These findings are contrary to the findings of Akinbo et al. (2007) and Akinbo et al. (2007) who in their separate studies found out that manual passive stretching alone significantly improved spasticity of wrist flexors, passive range of motion of the wrist as well as hand function in children with spastic cerebral palsy with and without the use of cryotherapy. They however both concluded that a combination of passive stretching, and cryotherapy produced better improvement in the spasticity of wrist flexors in children with spastic cerebral palsy [25,26]. The reason for lack of significant improvement in spasticity of wrist flexors and knee flexors following 10 weeks of land-based exercises in this present study could not be ascertained but there may be needed to go further beyond 10 weeks to get a significant improvement. Comparison between the experimental and control groups revealed no significant difference in the change in spasticity of all tested muscle groups. This implies that either of the two forms of intervention can be adopted in the management of spasticity in children with cerebral palsy. However, considering the result of this study which had earlier shown that aquatic intervention significantly improved the spasticity of all the tested muscle groups including those of wrist flexors and knee flexors which were not significantly improved by land-based exercises after 10 weeks, the beneficial pendulum might as well be tilted towards the aquatic exercise intervention when there is need for clinical decision making in the management of a patient presenting with spasticity of all the tested muscle groups in this study.
Conclusion
Aquatic exercise intervention is effective in inhibiting spasticity in children with Cerebral Palsy and should be an intervention of choice when considering affectation of all muscles of both upper and lower extremities.
References
- PJ Flett (2003) Rehabilitation of spasticity and related problems in childhood cerebral palsy. J Paediatr Child Health 39 (1): 6–14.
- LR Scheker, SP Chesher, S Ramirez (1999) Neuromuscular electrical stimulation and dynamic bracing as a treatment for upper-extremity spasticity in children with cerebral palsy . J Hand Surg Br. 24 (2): 226–232.
- Bar On L, Molenaers G, Aertbeliën E, Van Campenhout A, Feys H, Nuttin B et al. (2015) Spasticity and Its Contribution to Hypertonia in Cerebral Palsy. BioMed Research International pp.317-327.
- Pin T, Dyke P, Chan M (2006) The effectiveness of passive stretching in children with spastic cerebral palsy. Dev Med and Child Neurol 48: 855-862.
- Damiano DL, Dodd K, Taylor NF (2002) Should we be testing and training muscle strength in cerebral palsy? Dev Med Child Neurol 44: 68–72.
- Dimitrijević L, Aleksandrovic M, Madic D, Okicic T, Radovanovic D, Daly D (2012) The effect of aquatic intervention on the gross motor function and aquatic skills in children with cerebral palsy. Journal of Human Kinetics 32: 167-174.
- Darrah J, Law M, Pollock N (2001) Family-centered functional therapy – a choice for children with motor dysfunction. Infants Young Child 13: 79–87.
- RR Young, AW Weigner (1987) Spasticity. Clin Orthop Relat Res pp. 50–62.
- MB Glenn, J Whyte (1990) The practical management of spasticity in children and adults Lea & Febiger, Philadelphia .
- Getz M, Hutzler Y, Vermeer A (2006) Effects of aquatic interventions in children with neuromotor impairments: A systematic Review of the Literature. Clinical Rehabilitation 20: 927-936.
- Chrysagis DN, Douka A, Nikopoulos M, Apostolopoulou F, Koutsouki D (2009) Effects of an aquatic program on gross motor function of children with spastic cerebral palsy. Biology of Exercise 2009 5(2): 13-25.
- Blohm D (2011) Effectiveness of Aquatic Interventions for Children with Cerebral Palsy: Systematic Review of Current Literature. J Aquatic Phys Ther 19(1): 19-29.
- Akinola BI, Gbiri CA, Odebiyi DO (2019) Effect of a 10-Week Aquatic Exercise Training Program on Gross Motor Function in Children With Spastic Cerebral Palsy. Global Pediatric Health 6: 1-7.
- Fragala Pinkham M, Haley SM, O’neil ME (2008) Group aquatic aerobic exercise for children with disabilities. Developmental Medicine and Child Neurology 50(11): 822–827.
- Russell DJ, Rosenbaum PL, Avery LM, Lane M (2002) Gross Motor Function Measure (GMFM-66 and GMFM-88) User’s Manual. London, MacKeith Press.
- A Friedman, M Diamond, MV Johnston, C Daffner (2000) Effects of botulinum toxin A on upper limb spasticity in children with cerebral palsy. Am J Phys Med Rehabil 79 (1): 53–59.
- Nottidge VA, Okogbo ME (1991) Cerebral palsy in Ibadan, Nigeria. Dev Med Child Neurol 1991; 33: 241-245.
- Iloeje SO, Ejike-Orji I (1993) Compliance by cerebral palsy (CP) patients attending a child neurology service in a developing country: A preliminary study. West Afr J Med 12: 1-5.
- Adekoje TO, Ibeabuchi MN, Lesi FE (2016) Anthropometry of children with cerebral palsy at the Lagos University Teaching Hospital. J Clin Sci 13(3): pp.96.
- Park ES, Park CI, Lee HJ, Cho YS (2001) The effect of electrical stimulation on the trunk control in young children with spastic diplegic cerebral palsy. J Korean Med Sci. 16: 347–350.
- Fragala MA, Goodgold S, Dumas HM (2003) Effects of lower extremity passive stretching: Pilot Study of Children and Youth with Severe Limitations in Self-mobility. Pediatr Phys Ther 15: 167–175.
- Jorgić B, Dimitrijević L, Lambeck J, Aleksandrović M, Okicić T, Madić D (2012) Effects of aquatic programs in children and adolescents with cerebral palsy: Systematic Review. Sport Science 5(2): 49-56.
- Declerck M (2010) Effect of Aquatic Intervention on the Gross Motor Function and Quality of Life of Children with Cerebral Palsy. Master thesis. Leuven: Faculty kinesiology and rehabilitation sciences, University of Edinburgh.
- Pin T, Dyke P, Chan M (2006) The effectiveness of passive stretching in children with spastic cerebral palsy. Dev Med and Child Neurol 48: 855-862.
- Akinbo SR, Tella BA, Onunla AB, Temiye EO (2007) Comparison of the effect of neuromuscular electrical stimulation and cryotherapy on spasticity and hand function in patients with spastic cerebral palsy. Nig Med Pract 51(6): 128–132.
- El Maksoud GM, Sharaf MA, Rezk Allah SS (2011) Efficacy of Cold Therapy on Spasticity and Hand function in Children with Cerebral Palsy. Journal of Advanced Research 2: 319-325.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...