Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4722
Short Communication(ISSN: 2637-4722) 
Anti-endomysial Antibody (EMA) Testing: More is not always better Volume 3 - Issue 3
Liza L de Haan1*, Ben A Semmekrot2 and Jos W J van der Stappen2
- 1Department of Pediatrics, Canisius Wilhelmina Ziekenhuis, The Netherlands
- 2Department of Medical Laboratory, Canisius Wilhelmina Ziekenhuis, The Netherlands
Received:December 09, 2021; Published:December 17, 2021
Corresponding author:Liza L de Haan, Canisius Wilhelmina Ziekenhuis, Department of Pediatrics. Nijmegen, The Netherlands
DOI: 10.32474/PAPN.2021.03.000164
Abstract
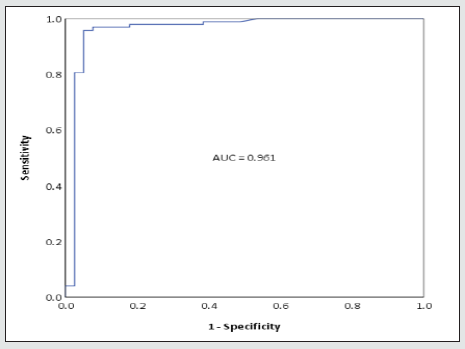
Celiac disease in children is a common gluten-induced autoimmune disease affecting the small intestine. Instead of invasive biopsy, the ESPGHAN (European Society for Paediatric Gastroenterology Hepatology and Nutrition) guideline offers an alternative diagnostic approach through the performance of IgA anti-tissue transglutaminase (IgA anti-tTG) levels along with anti-endomysial antibody (EMA) testing to confirm the diagnosis. According to the guideline, EMA testing is recommended when levels of IgA antitTG are > 10 times ULN. We studied the additional value of the performance of EMA testing in these cases with a retrospective database (N = 137, years 2012-2021). ROC curve analysis was performed to compare IgA anti-tTG levels with EMA result (positive/ negative), with EMA test as reference standard. High specificity rates were found (97.4- 100%) when IgA anti-tTG levels were > 25 times ULN.
Conclusion: EMA results are fully compatible with high levels of IgA anti-tTG (> 25 ULN) in the diagnostic approach of children with suspected celiac disease. Despite the recommendations of the guidelines, the performance of additional EMA tests, apart from IgA anti-tTG, is not always necessary. A short critical review shows that there is room for improvement
Keywords:Celiac disease; IgA anti tTG; Endomysial Antibody; ESPGHAN guideline
Introduction
Celiac disease in children is a common gluten-induced autoimmune disease affecting the small intestine (lifetime prevalence 1% [1]). Although an intestinal biopsy remains the gold standard to confirm the diagnosis, the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) guideline [2] offers an alternative approach for selected patients with the following characteristics:
a) clinical and morphological symptoms or/and at risk because of positive human leukocyte antigen (HLA) types DQ2/DQ8,
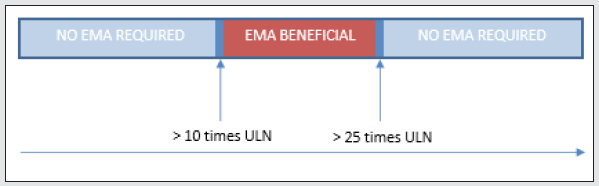
b) elevated IgA anti-tissue transglutaminase (IgA anti-tTG) results (> 10 times the upper limit of normal (ULN)) and
c) An additional positive anti-endomysial antibody (EMA) test. In these patients, the diagnosis is confirmed if symptoms resolve on a gluten-free diet and serologic tests revert to normal.
In this letter, we question the additional value of EMA testing in children with suspected celiac disease, when IgA anti-tTG levels are already performed and elevated. According to the ESPGHAN guideline, EMA testing should be performed when IgA anti-tTG levels are > 10 times the ULN (in our hospital 20 Chemiluminescent Units (CU)). In the ten years of performing these diagnostics (Quanta Flash human- tTG IgA Inova, EMA Indirect Immunofluorescence Test (IFT)) in children with suspected celiac disease in our hospital, interestingly, we noticed no additional diagnostic value of EMA when IgA anti-tTG levels were moderately elevated (around > 20 times ULN (> 400 CU)). EMA results turned out to be fully compatible with the upper levels of our collected data range of IgA anti-tTG levels. In those cases, the additional value of EMA for diagnosing celiac disease can indeed be questioned. Hypothetically, a cut- off value can be established whether or not to perform an EMA test, thus reducing the amount of diagnostic tests. This approach prevents unnecessary rise of healthcare costs (incidence x€50 per annum). Also, in some laboratories, EMA testing is still performed with monkey oesophageal tissue. Since animals use in medical science should be restricted to the minimum, critical review of this guideline is essential.
To (further) examine this hypothesis, a retrospective database was set up including children (< 18 years) suspected of celiac disease with available serologic IgA anti-tTG and EMA, in the years 2012- 2021. Only cases with a positive IgA anti-tTG level (≥ 20 CU) were included. Levels of IgA anti-tTG were compared with those of semi-quantitative EMA testing (positive/negative). To establish optimal cut- off value, ROC curve analysis was performed with EMA testing as reference standard. Specificity was determined for the upper limit of IgA anti-tTG. We also checked whether a biopsy was performed (data not shown). A total of 137 cases were included. Levels of IgA anti-tTG ranged from 20-64,300 CU. 98 (72%) of EMA results were positive. In general, EMA was performed less than 1 month apart from the initial IgA anti- tTG level, always in a different sample. In all cases where the guideline recommended a biopsy, the pathologic reports were consistent with IgA anti-tTG levels (data not shown). As ROC analysis shows (Figure 1), high specificity rates (97.4-100%) were found when IgA anti-tTG levels were 530-64,300 CU (> 25 times ULN). In these cases, EMA testing appears to have no additional value and can therefore be avoided. The total number of cases with IgA anti-tTG levels > 25 times ULN amounted 80 (58%). Because of lower specificity rates (< 95%) for IgA anti-tTG levels between > 10 and < 25 times ULN, we recommend EMA testing in these cases (Figure 2), in accordance with the ESPGHAN guideline.
In conclusion, despite the recommendations of the guideline, we found no additional value of EMA testing when IgA anti-tTG levels were > 25 times ULN. In those cases, the diagnosis celiac disease can be safely made together with the clinical symptoms and improvement on a gluten-free diet. Therefore, we advise to be more critical on performing EMA testing and validate the use of additional testing within your own organization as shown in this letter.
References
- Gujral N, Freeman HJ and Thomson AB (2012) Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol 18(42): 6036-6059.
- Husby S, Koletzko S, Korponay Szabó I, Kurppa K, Mearin ML, et al. (2020) European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J Pediatr Gastroenterol Nutr 70(1): 141-156.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...