Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4722
Case Report(ISSN: 2637-4722) 
A Neonate With Complete Congenital Heart Block Delivered Via Ex Utero Intrapartum Treatment (Exit) Procedure: A Case Report Volume 4 - Issue 2
Everett Jules V Deparine1, Jane D Estrera-Manlatican1, Shien Mariz L Gono1, Sheldon P Paragas1,2,3, Mae Anne C Valeroso1,3 and Genelynne J Beley1,3,4*
- 1Southern Philippines Medical Center, Department of Pediatrics, Davao City, Philippines
- 2Southern Philippines Medical Center, Section of Pediatric Cardiology, Davao City, Philippines
- 3Davao Medical School Foundation, Inc, College of Medicine, Department of Pediatrics, Davao City, Philippines
- 4Davao Medical School Foundation, Inc, Department of Biochemistry, Davao City, Philippines
Received: February 17, 2023 Published: February 22, 2023
Corresponding author: Genelynne J Beley, Southern Philippines Medical Center, Department of Pediatrics, Davao Medical School Foundation, Inc, College of Medicine, Department of Biochemistry, Davao City, Philippines
DOI: 10.32474/PAPN.2023.04.000183
Abstract
Congenital heart block (CHB) is a rare condition affecting newborns and infants. There is a delay or a complete obstruction in the electrical signals coordinating the contractility of the heart chambers [1] that presents fetal bradycardia. Most common cause of CHB is associated with maternal autoimmune disease, particularly systemic lupus erythematosus (SLE) [2]. However, CHB can occur in infants without an underlying cause. The diagnosis is made through physical examination, electrocardiogram, and echocardiogram. Presenting a case of a preterm neonate diagnosed with CHB on congenital scan during a prenatal check-up. Fetal bradycardia was noted hence referred to a pediatric cardiologist. Fetal 2D Echo revealed a complete congenital heart block hence the mother was worked up for SLE and confirmed with a positive ANA, Anti-SSA (Ro) and Anti-SSB (La). Pre-operatively, a multidisciplinary conference was convened with specialists from Newborn Medicine, Pediatric Cardiology, Electrophysiology, Thoraco-Cardiovascular Surgery, Obstetrics, Perinatology, Rheumatology and Anesthesiology. Everyone agreed to the proposed Ex Utero Intrapartum Treatment (EXIT) procedure. A scheduled primary cesarean section under general anesthesia was done 36 weeks AOG. Upon delivery, EXIT procedure, epicardial pacemaker insertion via median sternotomy, and left femoral artery cannulation were performed and the patient was further monitored at the Neonatal ICU. She stayed for 48 days, developed ventilator-associated pneumonia but eventually discharged improved. Long term plan for the patient includes frequent cardiac evaluation, growth, and development monitoring. During her follow-ups she was noted to have ASD (atrial septal defect) on diuretics with good compliance and is thriving well.
Keywords: Congenital heart block; ex utero intrapartum treatment; pacemaker
Introduction
Regulation of the normal and rhythmic pumping action of the heart muscle is vital in maintaining adequate circulation throughput the body. Congenital heart block (CHB), or atrioventricular block (AVB), is caused by a disruption in the conduction of electrical impulse that supports the function of the heart. The severity and prognosis of these conduction abnormalities differ among affected individuals.
Case Description
This is a case of a AS, a female newborn from Davao City, Philippines with fetal bradycardia initially noted prenatally. The patient was born to a then 30-year-old G3P2 mother with no known maternal illness but with a history of maculopapular rash on the lower extremities during the first trimester. On the 28th week of fetal life, AS was noted bradycardic at 60bpm, during a prenatal check-up. Congenital Anomaly Scan and Fetal doppler ultrasound revealed normal anatomic structures, good somatic movements and a persistently bradycardic fetus. Considerations at this time, were fetal distress, cord compression, structural problems, and congenital complete heart block. Further workups excluded other causes and heart block was highly suspected. The patient was first seen by the attending pediatric cardiologist at 31 weeks age of gestation. Comprehensive fetal echocardiography was done and showed normal anatomic structures with atrioventricular dissociation (Complete atrioventricular block/ fetal 3rd degree AV block).
Atrial rate was normal at 180 per minute but ventricular rate was low at 66 per minute. Chamber sizes were normal for age with good ventricular function and contractility indicating absence of heart failure. Pericardial effusion was not observed indicating no signs of hydrops fetalis. Parents were advised close monitoring and followup, as well as referral to the Perinatology/high-risk pregnancy specialist. The mother was worked up for a possible autoimmune disease and was referred to Southern Philippines Medical Center (SPMC) High Risk Pregnancy Clinic for closer monitoring of the pregnancy. The mother, though asymptomatic, was worked-up for autoimmune disease with the following findings of positive ANA titers at 1:320; both Anti-SSA (Ro) and Anti-SSB (La) were positive at 200u/mL, confirming a diagnosis of subclinical Systemic Lupus Erythematosus. The mother was referred to Adult Rheumatology. She was started on Prednisone 10mg/dose, Hydroxychloroquine 200mg/dose, and Calcium Carbonate and Iron supplements on the 34th week AOG [3].
At 33 weeks fetal life, repeat fetal 2D-echocardiography showed dissociation (Fetal Complete AV Block) with atrial rate of 176 per minute and ventricular rate of 63-65 per minute. Chamber sizes remained normal for fetal age with normal Z scores. Ventricular wall motion, contractility, and systolic function remained good at this point with no signs of feal heart failure despite the bradycardia. Signs of hydrops fetalis was not observed. Patient had good activity intrauterine, hence closer monitoring was continued. During this time a multidisciplinary meeting was called with Pediatric Cardiology, Neonatology, Perinatology, Electrophysiology, Rheumatology, Anesthesiology and Thoraco-cardiovascular Surgery (TCVS) to plan out the management. It was resolved that the termination of pregnancy via EXIT (Ex-Utero Intrapartum) procedure will be done once the patient reaches term and for permanent pacemaker insertion (PPI). At 35 weeks and 2 days fetal life, the patient was evaluated by a repeat fetal 2D-echocardiography.
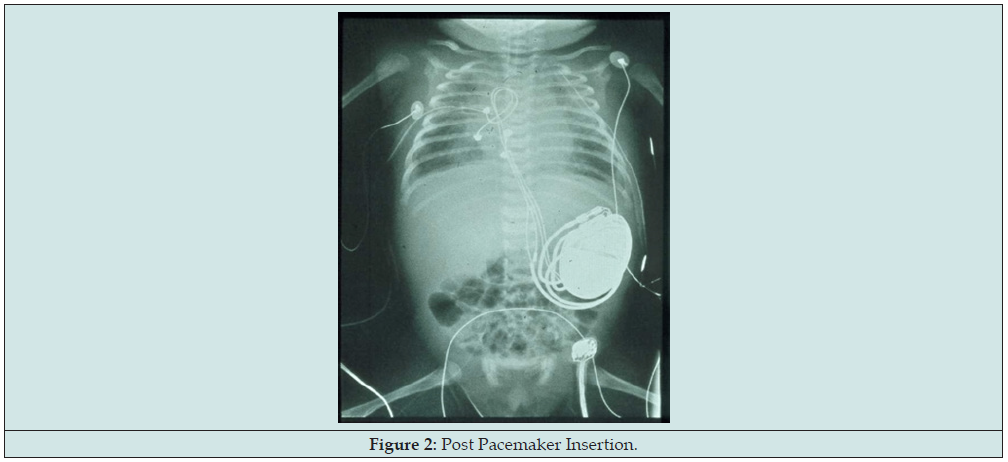
Fetal cardiovascular study still showed atrioventricular dissociation, this time ventricular rate now noted at 50 per minute. Atrial rate was normal at 136 per minute. Chamber sizes remained within normal limits for fetal age with normal Z score indicating absence of heart failure despite deterioration of fetal heart rate. Ventricular wall motion, contractility, and systolic function remained good. Still no signs of hydrops fetalis noted at this point. But on the 36 weeks’ gestation, drop in the fetal heart rate was noted, now to 50bpm hence the mother was immediately admitted for the proposed EXIT procedure and AS’ pacemaker insertion. Primary cesarean section under general anesthesia was done at 36 weeks AOG. Upon delivery, EXIT procedure, epicardial pacemaker insertion via median sternotomy, and left femoral artery cannulation were performed and the patient was then transferred to the Neonatal ICU (Figures 1 & 2). Patient’s APGAR score is 2, 7 with a maturity rating of 36 weeks AOG. Pertinent physical examination revealed a bradycardic and pale newborn, no gross deformities, no murmur, no hepatomegaly, no cyanosis with cold and fair pulses [4].
Discussion
Fetal bradycardia and Complete Congenital Heart Block (CHB) or Complete Atrioventricular Block (CAVB), three etiologic categories are considered: structural, non-structural cardiac defects and idiopathic. In 14-42% of cases with CCHB/CAVB found to have structural heart defects. Among the structural cardiac defects, primary considerations are Transposition of Great Vessels and Atrioventricular Septal defect. Due to its anatomical malformations, conduction systems of sinus and atrial nodes are affected that would present as first-degree heart block to a complete AV block. However, on the congenital scan and fetal 2d Echo of this patient, no noted gross cardiac anomalies. Considerations are narrowed down to non-structural causes and idiopathic. CCHB/CAVB that are caused by Autoimmune are found in 56-90% of cases. Perinatally complete AV block is detected in the background of maternal diagnosis of autoimmune disease such as SLE with positive antibodies. Maternal anti-Ro/SSA or anti-La/SSB antibodies passively crosses the placenta via the trophoblast and bind to L-type calcium channels in fetal cardiac muscles, specifically those in the AV node, and reversibly inhibit these channels’ current [5].
This results in inflammation, calcification, and fibrosis of the conduction tissue, leading to irreversible damage, even in structurally normal hearts. Idiopathic CAVB is associated with genetic abnormalities that have no relationship with autoimmune disease and in a structurally normal heart, however in this case mother is confirmed to have autoimmune disease with positive ANA, anti-Ro/SSA or anti-La/SSB antibodies hence idiopathic cause is unlikely. The cardiac conduction system of the heart is a network of specialized fibers that control the rhythmic and coordinated contractions of the heart chambers. The Sino-atrial node, the heart’s pacemaker is responsible for transmitting electrical signals and spread throughout the atria then travels to the AV node, before being transmitted to the ventricles via the AV bundle and Purkinje fibers. This results to coordinated contractions of the atria and ventricles pumping blood throughout the body. Abnormalities in the cardiac conduction system can lead to various heart rhythm disturbances, such as bradyarrthymias as manifested by AS. Blockage of impulses from the SA node allows subsidiary pacemakers to fire such as the AV nodal junction in this case [6].
Etiology
Congenital heart block is a rare condition, with an estimated incidence of 1 in 15,000 live births (Kahn, 2011). However, the incidence may be higher in mothers with autoimmune diseases such as SLE, with a reported incidence of 1 in 12 (Gharavi, 2010). One of the most significant causes of congenital heart block is exposure to certain medications during pregnancy, particularly anti-inflammatory drugs known as nonsteroidal anti-inflammatory drugs (NSAIDs) and hydroxychloroquine. These medications can cross the placenta and cause damage to the developing fetus’s electrical system. The occurrence of congenital heart block (CHB) in infants born to mothers with a history of nonsteroidal antiinflammatory drug (NSAID) intake during pregnancy has been a topic of concern in recent years. Studies have shown an increased risk of CHB in these infants whose mothers used NSAIDs during the first trimester of pregnancy, particularly those who used the drug for a prolonged period (Elliott et al., 2011; Hoppenbrouwers et al., 2017). The mechanism by which NSAIDs may cause CHB is thought to involve the inhibition of prostaglandin synthesis, which is essential for proper cardiac development during the early stages of pregnancy (Elliott et al., 2011). It is important to note that the overall risk of CHB in infants born to mothers who took NSAIDs during pregnancy is still relatively low, with a reported incidence of around 1 in 1,000 live births (Hoppenbrouwers et al., 2017).
However, given the potential risks associated with NSAID-use during pregnancy, it is important for pregnant women and their healthcare providers to weigh the potential benefits and risks of NSAID use, and to consider alternative treatment options whenever possible (American College of Obstetricians and Gynecologists, 2019). The relationship between hydroxychloroquine intake, however, during pregnancy and the development of congenital heart block is a topic of ongoing research. In this case, the mother had hydroxychloroquine upon diagnosis of SLE. According to a study published in the Journal of American Medical Association (JAMA) in 2021, the use of hydroxychloroquine during pregnancy was not associated with an increased risk of congenital heart block or other cardiac malformations in infants. However, the study also noted that the sample size was small and further research is needed to confirm these findings.
Another study from the Journal of Rheumatology in 2020, found that the use of hydroxychloroquine in pregnant women with systemic lupus erythematosus (SLE) was not associated with an increased risk of congenital heart block in their offspring. It is important to note that hydroxychloroquine is a category C pregnancy drug, which means that it may not be safe for use during pregnancy and should only be used if the potential benefit outweighs the potential risk to the fetus. It is important to consult with an Obstetrician before taking any medication during pregnancy [7].
Another possible cause of congenital heart block is an autoimmune disorder, such as Lupus which the mother of our index case recent diagnosis during the present pregnancy. In these cases, the mother’s immune system mistakenly attacks the baby’s developing electrical system. The exact mechanism by which SLE causes congenital heart block is not fully understood, but it is believed to be related to the presence of anti-Ro and anti-La antibodies, which are commonly found in women with SLE who have congenital heart block in their offspring. An in our case, the mother was noted with significant Anti-Ro and Anti-La antibodies at >200.00 U/ml which gave us a positive result. In Nelsons Textbook of Pediatrics (2019), in vitro studies suggest that during cardiac development via apoptosis, Ro and La antigens may be exposed on the surface of cardiac cells in the proximity of the atrioventricular node, making the antigens accessible to maternal autoantibodies. Binding of these antigens and autoantibodies induce a local immune response, resulting in fibrosis within the conduction system as well as more extensive disease in fatal cases. In the skin, exposure to ultraviolet light results in cell damage and the subsequent exposure of Ro and La antigens, inducing a similar local inflammatory response that produces the characteristic rash [8].
It is recommended that all pregnant women undergo screening with fetal echocardiography performed by a pediatric cardiologist on a weekly basis from 16-26 weeks of gestation, and then biweekly through 34 weeks. The time of greatest vulnerability is typically between 18-24 weeks. If an abnormal heart rate is detected during monitoring, and if fetal echocardiography confirms a conduction defect, it is recommended that the mother be screened for anti-Ro and anti-La antibodies. It is important to note that not all women with SLE will have a child with congenital heart block, and that other factors such as genetics and environmental exposures may also play a role. Pregnant women with SLE should be closely monitored by a maternal-fetal medicine specialist and a pediatric cardiologist to minimize the risk of congenital heart block in their offspring. In some cases, congenital heart block may also be caused by a genetic disorder or mutation. Mutations in the SCN5A gene have been linked to a form of congenital heart block known as Brugada syndrome. It is important to note that in many cases, the cause of congenital heart block is unknown. However, identifying the cause can help guide treatment and management decisions, and may also provide important information for the patient’s family members [9].
Diagnosis
The diagnosis of congenital heart block is typically made through examination, electrocardiogram, and echocardiogram. (Kahn, 2011). A physical examination may reveal a slow or irregular heart rate, as well as a heart murmur. In congenital heart block, the ECG may show a slow heart rate and a prolonged PR interval, which is the time it takes for the electrical impulse to travel from the atria to the ventricles. The PR interval is usually greater than 200 milliseconds in congenital heart block. Additionally, the QRS complex, which represents the electrical activity in the ventricles, may be widened, indicating a delay in the electrical conduction through the bundle of His. For the patient, the ECG after delivery prior the insertion of the Pacemaker was bradycardic at 73 bpm, and it also showed prolonged PR interval. Other ECG findings that may be seen in congenital heart block include:
a) A prolonged QRS complex, which is the time it takes for the electrical impulse to travel through the ventricles.
b) A right bundle branch block (RBBB) or left bundle branch block (LBBB) pattern, indicating a delay or block in the electrical conduction in the bundle of His.
c) A complete heart block, in which the atria and ventricles beat at different rates. However, it’s important to note that ECG alone may not be sufficient for a definitive diagnosis of congenital heart block. It is recommended that a fetal echocardiogram or a neonatal echocardiogram is done to confirm the diagnosis [10].
Echocardiography can reveal several significant findings, including:
a) Structural heart defects such as ventricular septal defect (VSD), atrial septal defect (ASD), and patent ductus arteriosus (PDA).
b) Left ventricular dysfunction: Patients with congenital heart block may have left ventricular dysfunction, can be diagnosed by echocardiography through the measurement of ventricular ejection fraction (LVEF) and the assessment of left ventricular systolic and diastolic function.
c) Right ventricular dysfunction: Patients with congenital heart block may also have right ventricular dysfunction. It can be diagnosed by echocardiography through the assessment of right ventricular size and function.
d) Conduction system abnormalities: Echocardiography can reveal conduction system abnormalities such as the absence or hypoplasia of the atrioventricular node or bundle of His. It is important to note that the echocardiogram findings in congenital heart block may vary depending on the specific type and severity of the condition, as well as the presence of other associated structural heart defects [11].
Management
Intrapartum management of congenital heart block involves close monitoring of the mother and the fetus during labor and delivery. The goal of management is to ensure the safe delivery of the baby and to minimize any potential complications. One important aspect of intrapartum management is monitoring of the fetal heart rate, which can be done using electronic fetal monitoring (EFM). This allows healthcare providers to detect any changes in the fetal heart rate that may indicate distress, such as bradycardia (a slow heart rate). If fetal distress is detected, the healthcare provider may need to take action to deliver the baby as soon as possible. Another important aspect in the management is the use of a specialized delivery technique known as an EXIT procedure. or ex utero intrapartum treatment procedure. It is used when a baby has a congenital heart block and requires intervention to establish or maintain a stable airway before delivery. This procedure is performed under general anesthesia and involves partially delivering the baby through the birth canal while keeping the umbilical cord intact to maintain fetal circulation. The baby is then stabilized and transferred to a neonatal intensive care unit (NICU) for further care.
This procedure was done in patient AS. In addition to electronic fetal monitoring and EXIT procedures, other management strategies may include the use of medications such as atropine to increase the heart rate, and the use of cesarean delivery if necessary to ensure the safe delivery of the baby. It is important to note that the management of congenital heart block requires a multidisciplinary approach involving obstetricians, maternal-fetal medicine specialists, pediatric cardiologists, and neonatologists. Definitive treatment for congenital heart block typically involves the use of a permanent pacemaker. With appropriate management, the prognosis for infants with congenital heart block is generally good, with an estimated survival rate of 85-95% [12].
Complications
The complications of congenital heart block can vary depending on the severity of the condition. Some of the most common complications include:
a) Heart failure: Infants with severe heart block may be at risk for heart failure. Electrical signals that are not properly conducted through the heart may result to ineffective pumping of blood by the heart. This can lead to a buildup of fluid in the lungs and other parts of the body. This results to heart failure. Additionally, if the heart rate is too slow, the heart may not be able to pump enough blood to meet the body’s needs, leading to heart failure.
b) Stroke: Infants with congenital heart block may be at an increased risk of stroke due to the irregularity of the heart rhythm.
c) Sudden Cardiac Death: Infants with congenital heart block may be at risk for sudden cardiac death if the heart block is not diagnosed and treated promptly.
d) Pacemaker dependence: Long-term use of a pacemaker may lead to pacemaker dependence, resulting for an individual to rely on the pacemaker for the rest of their life.
e) Pacemaker infection: There is a small risk of infection with pacemaker. This may require removal of the device.
f) Lead displacement: The leads of the pacemaker are responsible for transmitting the electrical impulses. It may become displaced and require repositioning.
g) Battery depletion: The battery of the pacemaker needs to be replaced periodically. It is important to note that these complications can vary based on the individual case and the severity of the condition. It is also important to follow the physician’s recommendations for monitoring and management to minimize the risk of complications [13].
Follow-up
The frequency of monitoring for infants with congenital heart block and a permanent pacemaker will depend on the recommendations of the treating physician. In general, infants with congenital heart block requires to undergo regular follow-up appointments with a pediatric cardiologist to monitor the function of the pacemaker and the overall health of the heart. During these appointments, the infant’s heart rate, rhythm, and the functioning of the pacemaker will be evaluated. The pacemaker will be checked for proper function and the battery life, lead integrity and threshold will be checked. In general, most pacemaker batteries have a lifespan of about 5-15 years, although some newer pacemakers have batteries with lifespans of up to 20 years. Depending on the status, the pacemaker may need to be reprogrammed or replaced. Additionally, the infant’s growth and development will be monitored to ensure that the pacemaker is still providing adequate support as the child grows. For the first few months, follow-up appointments may be more frequent, usually every 34 weeks, and then may be spaced to every 3-6 months, depending on the patient’s condition and the physician’s discretion. It is important to follow the physician’s recommendations for monitoring and to keep all scheduled appointments to ensure the best possible outcome for the infant [14].
Prognosis
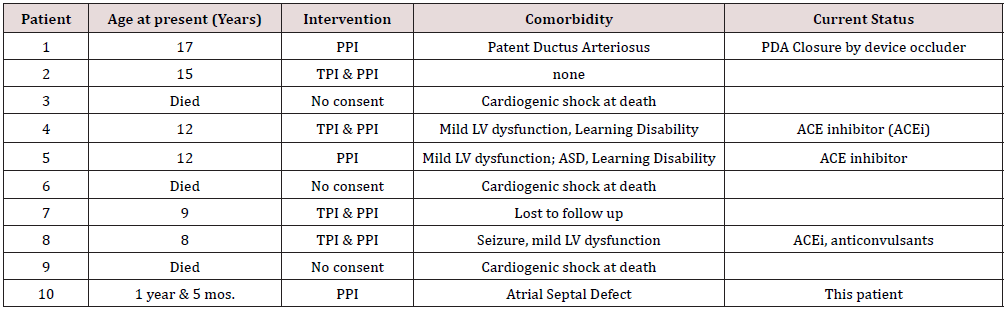
It is difficult to provide a specific percentage for the prognosis of infants with complete congenital heart block as it can vary depending on the severity of the condition and the promptness of diagnosis and treatment. However, it is generally considered that infants with mild heart block and no structural heart defects will have good prognosis with appropriate management and monitoring. However, infants with severe heart block or structural heart defects may have a poor prognosis, including an increased risk of heart failure, stroke, and death. Factors such as the type of congenital heart block, the underlying causes, and the presence of other associated heart defects all play a role in determining the overall prognosis. The survival rate for infants with complete congenital heart block is generally lower compared to those with other types of congenital heart block. Other factors such as the presence of structural heart defects and the promptness of diagnosis and treatment can also affect the survival rate in individual cases. From a local data (Table 1), three (3) of the ten (10) patients with complete heart block died of cardiogenic shock in the absence of a pacemaker [15].
Table 1: Complete Congenital Heart Block (CCHB) cases in Davao City, Philippines by a Single Pediatric Cardiologist’s Experience.
Conclusion
Complete Congenital Heart Block (CCHB) is a rare condition that can occur due to several causes, including exposure to certain medications such as Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Hydroxychloroquine during pregnancy. Another possible cause of CHB is an autoimmune disorder, such as Systemic Lupus Erythematosus (SLE), which the mother of this case had recently been diagnosed with. In autoimmune etiology, the mother’s immune system affects the developing fetal cardiac electrical system, which is believed to be related to the presence of anti-Ro and anti-La antibodies. It is recommended that all pregnant women undergo regular fetal echocardiography to monitor the health of the developing fetus and detect any potential abnormalities. If an abnormal heart rate is detected and confirmed by fetal echocardiography, the mother should be screened for anti-Ro and anti-La antibodies. In some cases, CHB may also be caused by genetic disorders or mutations. In order to minimize the risk of CHB in offspring, pregnant women with SLE should be closely monitored by a maternal-fetal medicine specialist and a pediatric cardiologist. It is important to note that the management of congenital heart block requires a multidisciplinary approach involving obstetricians, maternal-fetal medicine specialists, pediatric cardiologists, and neonatologists. Untreated congenital heart block can be life-threatening, especially in infants and young children. Early detection and treatment are crucial in improving the prognosis of congenital heart block.
References
- Congenital Heart Block. American College of Cardiology. Washington DC, USA.
- Congenital Heart Block. American Heart Association. Dallas, USA.
- Kahn AM (2011) Congenital heart block. Orphanet Journal of Rare Diseases 6(1): 1-15.
- Gharavi AG, Petri M (2010) Neonatal lupus erythematosus. Lupus 19(4): 389-396.
- Chen L, Han X, Li Y, Li X, Chen L, et al. (2018) Hydroxychloroquine or azathioprine for preventing maternal morbidity and congenital heart block in neonates born to mothers with systemic lupus erythematosus: a systematic review and meta-analysis. Rheumatology (Oxford, England) 57(7): 1161-1169.
- Costedoat-Chalumeau N, Amoura Z, Seguin C, Le Guern V (2003) Congenital heart block in neonates born to mothers with anti-SSA/Ro antibodies: a prospective multicenter study. Journal of the American College of Cardiology 42(11): 1894-1901.
- Gersony WM, Hayes CJ, Driscoll DJ, Gershon AA (1983) Outcome of infants with congenital heart block. The Journal of Pediatrics 103(6): 817-826.
- Kugler JD, Silverman NH, Gersony WM (2007) Diagnosis and management of congenital heart block. Circulation 116(7): 849-861.
- Melim C, Pimenta J, Areias JC (2022) Congenital Atrioventricular Heart Block: From Diagnosis to Treatment. Port J Cardiol 41(3): 231-240.
- Meroni PL, Doria A, Shoenfeld Y (2002) Lupus and the heart. Autoimmunity reviews 1(4): 259-263.
- Nelson TE, Greer LG, Saade GR (2018) Congenital heart block. The Lancet 392(10157): 1289-1300.
- Nelson Textbook of Pediatrics. (21st ed.) Philadelphia, PA: Elsevier, USA.
- Prenner SB, Gersony WM, Ritter SB, et al. (1991) Echocardiographic findings in congenital complete heart block. J Am Coll Cardiol 17(3): 711-719.
- Sable CA, Gersony WM, Ritter SB, et al. (1995) The natural history of congenital complete heart block: a multicenter study. J Am Coll Cardiol 26(7): 1655-1661.
- (2019) The EXIT procedure. Children’s Health, Texas, USA.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...