Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Case Report(ISSN: 2641-1709) 
Use of Temporoparietal Flap and Split Skin Grafting of Donor Area to Salvage Cochlear Implant – Novel Experience from A Tertiary Hospital in India Volume 6 - Issue 4
Anand R1*, Shyam Kalyan N2, Jaisy J Vikkath3 and Aruchamy M4
- 1Senior Consultant, Department of Cochlear Implant Surgery, PSG Super Specialty Hospital, India
- 2Associate Consultant, Department of ENT, PSG Super Specialty Hospital, India
- 3Senior Resident, Department of ENT, PSG Super Specialty Hospital, India
- 4Senior Consultant, Department of Plastic Surgery, PSG Super Specialty Hospital, India
Received: May 04, 2021 Published: May 18, 2021
Corresponding author: Anand R, Senior Consultant, Department of Cochlear Implant Surgery, PSG Super Specialty Hospital, India
DOI: 10.32474/SJO.2021.06.000241
Abstract
The management of cochlear implant exposure can be very challenging especially in the pediatric population where reconstructive options are limited. Various techniques comprising conservative and surgical methods have been used to prevent explanation with variable success rates. We describe the use of the temporoparietal fascial flap (TPFF) for this purpose due to its wide coverage and tension-free closure. We present the case of a child who had implant exposure 3 months after the surgery. He was managed conservatively initially. When this failed restoring of the wound was done. However, this also was not successful. Then we tried coverage with TPFF and split-thickness skin grafting of the TPFF donor site which succeeded. Our experience provides evidence that TPFF can be used for the coverage of post aural wound in cases of implant exposure and when combined with a splitthickness skin graft (STSG) of the donor site, it can further increase the success rates. The STSG of the donor site ensures a tensionfree closure in comparison with primary closure which would put further pressure on the TPFF.
Keywords:TPFF: Temporoparietal Fascial Flap; STSG: Split-Thickness Skin Graft
Introduction
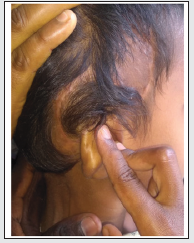
One of the major soft tissue complications following cochlear implantation is the skin flap necrosis. It has been reported in the literature with an incidence of 1.08% to 8.2%. Skin flap necrosis leading to implant exposure may necessitate its removal even. Several methods have been described in the literature to salvage an exposed implant. The temporoparietal fascial flap (TPFF) is an excellent option for reconstruction of device site soft tissue dehiscence when local wound care and primary closure are not sufficient. The flap can prevent the implantation of a functional implant. It provides a well vascularized thin pliable tissue that is readily accessible locally. We describe our experience in using TPFF combined with STSG of the donor area to salvage a functional implant a child with implant exposure (Figure 1).
Case History
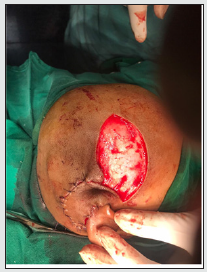
A 4-year-old boy who underwent right-sided cochlear implantation with a Nucleus 22 channel cochlear implant presented to us after 2 months with thinning of overlying skin. On examination, a 0.5 cm X 0.5 cm area of full-thickness necrosis exposing the underlying receiver-stimulator was seen. The skin adjoining the site of exposure was thin and delicate. Regular cleaning and dressing of the wound were done under strong antibiotic cover. Conservative management failed. After a complete anesthetic workup, he was taken for resuturing under general anesthesia. The margins of the wound were freshened after the removal of unhealthy thin tissue over the implant. After sufficient undermining, resuturing was done without tension. The dressing was done meticulously, and the child was discharged with strong oral antibiotics. Change of dressing on the fifth postoperative day showed a healthy cover over the implant and good healing. The child presented again after 3 weeks with exposure of the implant over the same site. There was a slightly larger area of necrosis over the device than before. This time the skin over the electrodes also had got thinned out. Under general anesthesia, the post aural wound was thoroughly debrided of all devitalized tissue. The device was checked for any biofilm growth. A ‘U’ shaped incision was made over the temporal area with its posterior limb in continuity with the original post aural incision. The incision was deepened till the deep temporal fascia and the temporoparietal flap of about 3 cm X 5 cm was raised off the fascia cephalic to caudal. The superficial temporal vessels on which the flap was based were preserved. The flap was rotated 180 degrees inferiorly over the implant and the post aural wound was closed. A split-thickness skin graft (STSG) was harvested from the thigh to close the defect over the donor site. Appropriately spaced slits were made on the graft using a scalpel (Figure 2). The graft was placed over the donor site on the scalp and sutured. On the fifth postoperative day, the dressing was changed. The post aural wound was dry, and the flap cover was healthy. The graft over the donor site was intact. On subsequent follow-up visits the post aural wound healed completely with good taking up of the STSG over the donor site..
Discussion
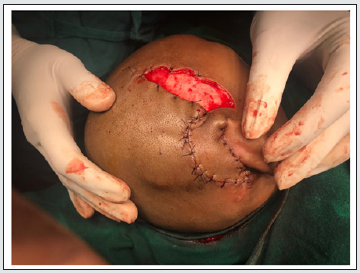
Major skin flap complications after cochlear implantation have been reported to have a frequency of 1.08% to 8.2%. Several methods have been described in the literature to salvage the implant in such cases. One of the first methods tried is conservative management with cleaning and dressing of the site with a good antibiotic cover. However, this method is often ineffective and may lead to explanation in many [1]. The first surgical treatment option for managing skin flap complications is wound debridement, excision of unviable tissues, an inspection of the device, and secondary suturing. The same must be performed under a strong intravenous antibiotic cover [2]. It has been postulated that for primary closure the defect should be less than 2 cm2 and those with areas between 2 and 25 cm2 need a flap cover [3]. Various flaps have been tried to provide a cover for the exposed cochlear implant with relative advantages and disadvantages. Some of those are the post aural pedicle flap, scalp rotational flap, pericranial flap, anterolateral thigh flap, pedicled temporalis muscle flap, and temporoparietal fascia flap [4]. Rotation of skin flap or dislocation of the transducer under healthy soft tissue has been attempted (Figure 3). Low et al performed rotational skin flap in 5 cases which succeeded in 2 and transposition of the device in another which succeeded. The 3 cases, which failed with rotational flap cover, were given transposition of the device and that succeeded [5].
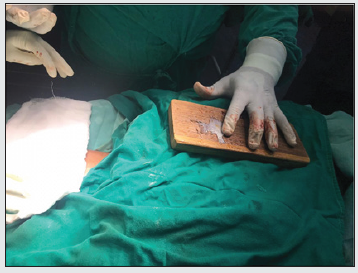
A 2-layer flap cover has been described which involves the skin and subcutaneous tissue as the external layer, and muscle and fascia as the internal layer. The technique was successful in 3 out of the 4 cases in which it was performed [6]. Surgeons have used free flaps also to cover the defect when the local flap conditions are poor or the defect too large [7]. Temporalis myofascial flap has been used by many authors to cover the defect arising post cochlear implantation. Some have advocated the use of anterolateral thigh flap or tissue expanders and perform a two-stage procedure [8]. The TPFF is based on the superficial temporal artery as pedicle. It has been used extensively in ENT, Head, and Neck surgeries to reconstruct the ear, eyelid, and nasal ala [9]. It has been used as a tunneled fascio-cutaneous hair-bearing flap for eyebrow reconstruction [10]. Previously 2 of our implant exposure cases were treated with local rotational skin flap cover but that failed in both. It was seen that such a cover did not afford sufficiently thick covering over the transducer leading to pressure necrosis eventually. The elasticity of the scalp is limited due to the underlying galea and pericranium. In our case, the TPFF with skin was rotated 180 degrees to cover the defect in the postauricular region in a tension-free manner (Figure 4). The donor area was covered with STSG taken from the anterior part of the thigh. Primary closure of the donor site of TPFF puts tension on the flap compromising the post aural reconstruction. So, we used the STSG. In a similar case, however in an adult patient, scalp defect over the cochlear implant was covered using TPFF and the donor site was covered with STSG harvested from the opposite side scalp [11]. Being an adult sufficient STSG could be harvested from the opposite side scalp. Because our patient was a small child, we used STSG from the thigh to obtain a large graft with enough surface area. TPFF has many advantages in this context. It is a pliable, versatile, and well-vascularized flap with sufficient surface area to cover post-aural defects. It is readily accessible, flexible, and has a wide rotation arc. It has been advocated for covering cochlear implant exposure because of its anatomical proximity and low donor site morbidity. The accompanying scalp defect can be covered using a local flap or an STSG (like in our case).
Conclusion
The TPFF is a relatively easy and quick option for the reconstruction of scalp defects causing implant exposure due to its anatomical proximity and low donor site morbidity. This can be combined with an STSG, harvested from the thigh, to achieve a tension-free closure of the donor site rather than primary suturing. This method can reduce explanations.
References
- Garcia-Valdecasas J, Jimenez-Moleon JJ, Sainz M, Fornieles C, Ballesteros JM (2009) Prophylactic effect of clarithromycin in skin flap complications in cochlear implants surgery. Laryngoscope 119(10): 2032-2036.
- Yu KC, Hegarty JL, Gantz BJ, Lalwani AK (2001) Conservative management of infections in cochlear implant recipients. Otolaryngol Head Neck Sur 125(1): 66-70.
- Leedy JE, Janis JE, Rohrich RJ (2005) Reconstruction of acquired scalp defects: an algorithmic approach. Plast Reconst Surg 116(4): 54-72.
- Karimnejad K, Akhter AS, Walen SG, Mikulec AA. The temporoparietal fascia flap for coverage of cochlear reimplantation following extrusion. Int J Pediatr Otorhinolaryngol 94: 64-67.
- Low WK, Rangabashyam M, Wang F (2014) Management of major post-cochlear implant wound infections. Eur Arch Otorhinolaryngol 1(9): 2409-2413.
- Wojciech Gawe, Michał Karlik, Łukasz Borucki, Joanna Szyfter-Harris, Maciej Wrobel (2016) Skin flap complications after cochlear implantations. Eur Arch Otorhinolaryngol 273(12): 4175-4183.
- Newman MI, Hanasono MM, Disa JJ, Cordeiro PG, Mehrara BJ (2004) Scalp reconstruction: a 15-year experience. Ann Plast
Surg 52(5): 501-506. - Geraghty M, Fagan P, Moisidis E (2014) Management of cochlear implant device extrusion: case series and literature review. J Laryngol Otol 128(Suppl 2): 55-58.
- Yamauchi M, Yotsuyanagi T, Yamashita K, Ikeda K, Urushidate S, et al. (2012) The reverse superficial temporal artery flap from the preauricular region, for the small facial defects. J Plast Reconstr Aesthet Surg 65(2): 149-155.
- Motomura H, Muraoka M, Nose K (2003) Eyebrow reconstruction with intermediate hair from the hairline of the forehead on the pedicled temporoparietal fascial flap. Ann Plast Surg 51(3): 314-318.
- Kang JK (2001) Scalp reconstruction using TPFF and STSG.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...