
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1709
Case Report(ISSN: 2641-1709) 
Primary Mucosal Malignant Melanoma of the Maxilla: A Case Report and Review of Literature Volume 5 - Issue 5
Garima Rawat1*, Hema Malini Aiyer2 and Anshuman Kumar3
- 1Department of Oral & Maxillofacial Pathology, Dharamshila Narayana Hospital, India
- 2Department of Pathology, Dharamshila Narayana Hospital, India
- 3Department of Surgical Oncology, Dharamshila Narayana Superspeciality Hospital, India
Received: January 18, 2021 Published: January 29, 2021
Corresponding author: Garima Rawat, Department of Oral & Maxillofacial Pathology, Dharamshila Narayana Hospital, India
DOI: 10.32474/SJO.2021.05.000224
Abstract
Primary mucosal malignant melanoma of oral cavity is a rare malignancy arising from the melanocytes. Mucosal melanomas of the head and neck comprise just over 1% of all melanomas and of these about 50% arise in the oral cavity. Oral mucosal melanomas are therefore rare, representing about 0.5% of oral malignancies and less than 0.01% of all oral biopsies. It tends to metastasize and locally invade tissues more than other malignant tumours of the oral cavity. It occurs commonly in the maxillary gingiva more frequently on the palate and lesser often in the mandibular gingiva. Here, we present a case of a mucosal malignant melanoma involving the maxillary bone along with the immunohistochemical profile of the neoplasm.
Keywords:Mucosal malignant melanoma; Maxilla; HMB-45
Abbreviations:WHO: World Health Organization; PET: Positron Emission Tomography; WLE: Wide Local Excision; MND: Modified Neck Dissection; AJCC: American Joint Committee on Cancer; CTLs: Cytotoxic T lymphocytes
Introduction
Mucosal malignant melanoma is a rare aggressive neoplasm arising from melanocytes which are derived from the neural crest. These melanocytes constitute the melanin pigment in the basal and suprabasal layers of the epithelium. Although most melanomas arise in the skin, they may also arise from mucosal surfaces [1,2]. Mucosal malignant melanoma has been described in virtually all sites and organ systems to where neural crest cells migrate. The World Health Organization (WHO) has defined mucosal malignant melanoma as a malignant neoplasm of melanocytes or of melanocyte precursors [3]. It is characterized by a proliferation of atypical melanocytes at the epithelial-connective tissue interface associated with upward migration into the epithelium and invasion of the underlying connective tissue. Mucosal malignant melanomas have been reported to show a higher incidence in Japan, India, and Africa than that in western countries. These melanomas tend to present at an advanced stage, are more aggressive, and usually present in the vertical (nodular) growth phase of the disease [2,4]. We present a rare case of Mucosal malignant melanoma involving primarily the maxilla and hard palate.
Case Report
A 57-year-old male presented to the outpatient department of a tertiary care cancer hospital with the chief complaint of a painless blackish swelling in the posterior maxillary region since 3 months. There was no past history of trauma, systemic illness or associated bleeding or pain. Intraoral examination revealed a nontender bluish- grey mass on the palatal mucosa, maxillary alveolus and buccal vestibule measuring approximately 5 cm in greatest dimension. The mass was pedunculated with a smooth surface and well‑defined margins. On palpation the lesion was slightly tender, soft in consistency; smooth textured, with evidence of bleeding on probing. Patches of melanosis were evident on the adjoining mucosal surfaces and gingiva of teeth. Based on the clinical findings, a provisional diagnosis of mucosal malignant melanoma involving maxillary alveolus and palate was made. A differential diagnosis of vascular neoplasms like hemangioma, melanoacanthoma and oral melanotic macule were considered. Further investigations were advised. Complete blood cell counts as well as biochemical analysis were insignificant and within normal limits. Positron emission tomography (PET) scan and contrast‑enhanced computed tomography imaging revealed a soft tissue enhancing lesion measuring 5.5 cm in greatest dimension involving the right maxillary vestibule and infiltrating the retromolar region and masticator space with possible erosion of maxillary alveolar process. Few metabolically active cervical lymph nodes on the right side at levels I and II were reported.
An incisional biopsy was done under local anesthesia, which showed sheets and islands of epithelioid melanocytes, arranged in an organoid and alveolar pattern. The cells had pale cytoplasm and large vesicular nuclei with prominent nucleoli. Occasional epitheloid cells and spindle shaped cells with cytoplasmic pigment were present. Morphologic features were consistent with Poorly Differentiated Malignancy – possibly a Mucosal malignant melanoma. Immunohistochemistry was advised for confirmation of diagnosis. The patient was taken up for surgery in the department of surgical oncology and was planned for partial maxillectomy along with Wide Local Excision (WLE) of buccal mucosa and palatal mucosa along with Modified neck dissection (MND) Type III of the ipsilateral side. The surgical resected specimen was sent to the pathology department for intraoperative margin status assessment, which were found to be sufficient and negative on histopathological examination. The specimen received in the pathology department measured 7.0x6.0x4.0 cm and included partial maxillectomy, WLE buccal mucosa, palatal mucosa and separate right MND type III (Figure 1A). Grossly the neck dissection specimen showed pigmented cervical lymph nodes at levels I and II (Figure 1B). Histopathological findings were consistent with the incisional biopsy findings with more areas showing epithelioid and spindle cell differentiation (Figure 2(A-D)). IHC was performed and the tumour showed strong cytoplasmic immunoreactivity for HMB-45 and Melan-A, and nuclear staining for SOX-10 (Figure 3(AC)). Based on the Morphologic & IHC features, a final diagnosis of Mucosal Malignant Melanoma with epithelioid and spindle cells was made.
Figure 1: A) Gross image of the main resected specimen showing the tumour involving maxilla; B) Gross pictures of the lymph nodes at level I and II showing pigment deposit.
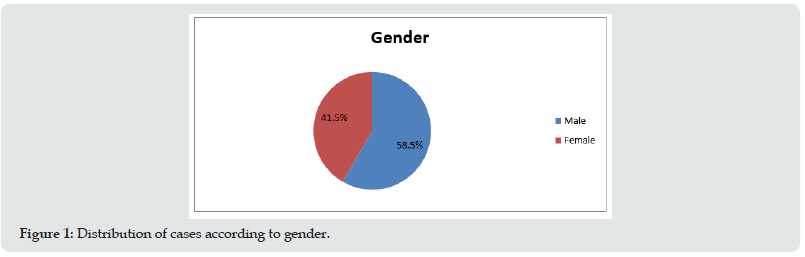
Figure 2: A) Photomicrograph showing sheets of spindle shaped cells showing melanin pigment deposition. (HE-40x); B, C) Photomicrograph showing spindle shaped cells and epitheloid cells showing abundant melanin pigment. (HE-400x); D) Photomicrograph showing decalcified sections showing tumour cells invading the maxillary bone. (HE-400x).
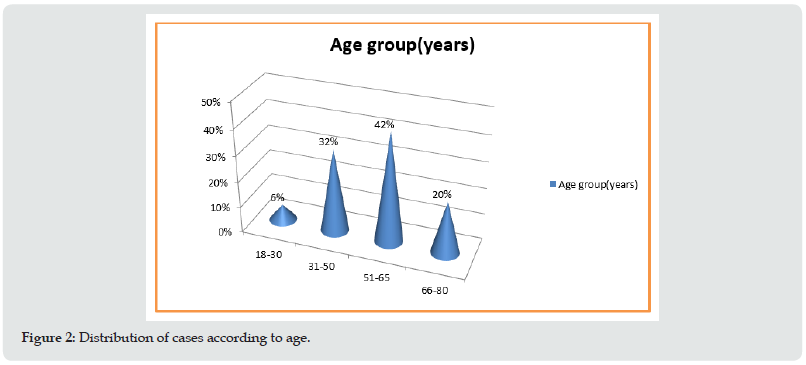
Figure 3: A) Photomicrograph showing strong cytoplasmic immunoreactivity for HMB-45. (IHC-400x); B) Photomicrograph showing strong cytoplasmic immunoreactivity for Melan-A. (IHC -400x); c) Photomicrograph showing strong nuclear immunoreactivity for SOX-10. (IHC-400x).
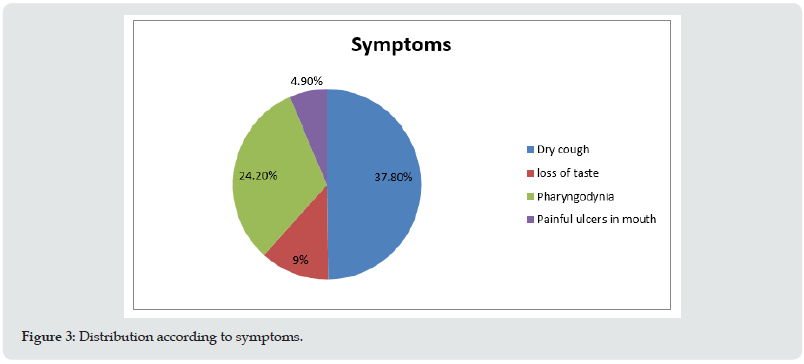
All surgical resection margins were uninvolved by tumour and the closest margin (medial margin) was 1.4cm from the tumour. Four cervical lymph nodes (out of thirty-nine lymph nodes examined) at Right levels Ia, Ib and IIa showed metastatic deposits of tumour, Extranodal extension was present at level Ib (Figure 4(a & b)). The lymph node ratio was 0.11 (04/39). The decalcified sections from mid maxillary bone showed invasion of the bone by tumour. The final pathologic TNM staging was done according to AJCC 8th edition staging guidelines for oral mucosal melanoma as under:
Figure 4: A) Photomicrograph showing tumour deposits in level Ia lymph nodes. (HE- 40x); B) Photomicrograph showing tumour deposits in level Ib lymph nodes. (HE- 100x).
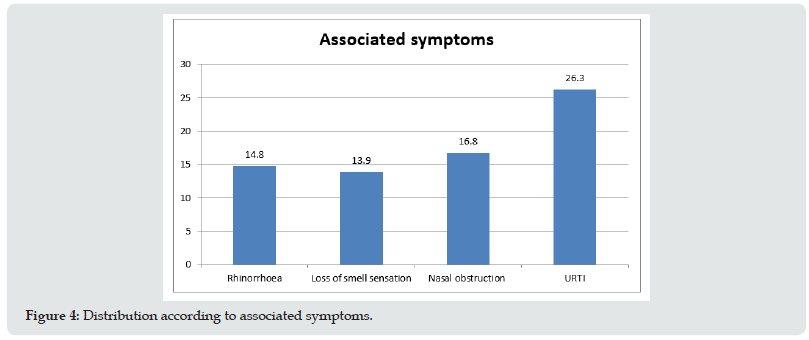
a) Primary Tumour (pT): pT4a:Moderately advanced disease. Tumour involving deep soft tissue and maxillary bone.
b) Regional Lymph Nodes (pN): pN1:Regional lymph node metastases present.
The patient was discharged and had an uneventful recovery. The patient is on follow up since then.
Discussion
Mucosal malignant melanoma was first described by Weber in 1859 and after that it was recognized as a distinct clinical entity and named as “melanotic sarcoma” by Lucke in 1869. This entity represents 0.5% of all neoplasms affecting the oral cavity. They arise in adults in the 5-6th decades of life. Very rare cases have been reported in children. In most large series there is a male predominance in a ratio of about 3:1. From various other studies in the past, the male-to-female ratio ranges between 1:1 and 2:1 [1- 4]. Eighty percent of oral melanomas arise on the palate, maxillary alveolus or gingiva as in the present case. Other sites include the mandibular gingiva, buccal mucosa, floor of mouth and tongue. These tumours present in two patterns:
a) Rapid appearance and enlargement of a pigmented lesion or
b) Preceded by pigmentation for a variable period of time. Oral pigmentations precede melanoma (for several months or years) in 30% of the cases.
The first symptoms of an oral melanoma identified by Berthelsen et al were those of asymptomatic swelling and occasional bleeding. Clinically, mucosal melanomas are usually asymmetric with irregular outlines. They may be black, grey, or purple to red, and rarely amelanotic. Typical lesions are composed of multiple or widespread areas of macular pigmentation with areas of nodular growth. Purely macular lesions may be seen but over 50% of lesions present as nodules or as a pigmented epulis. Because most melanomas are painless in their early stages, the diagnosis is unfortunately often delayed until symptoms resulting from ulceration, growth and / or bleeding are noted. Many reports document longstanding ‘melanosis’ before the onset of nodular lesions, with a history of up to 10 years [5]. Oral lesions are usually advanced at presentation with up to 75% of patients having metastases to cervical lymph nodes, and 50% with distant metastases, usually to the lung or liver [6-8]. In the case being discussed here, there was a short duration history, no pain or associated bleeding from the mass. However, in spite of the short duration the cervical lymph nodes had metastatic tumour deposits. Clinically, the tumours are classified into five types.
a) Pigmented nodular.
b) non pigmented nodular.
c) Pigmented macular.
d) Pigmented mixed and
e) non pigmented mixed. Melanoma of the oral cavity may occur with or without a radial growth phase [1,2,4].
Greene et al. suggested the following criteria for a lesion to be considered as malignant melanoma of the oral cavity:
A. Demonstration of malignant melanoma both histologically and clinically.
B. The presence of junctional activity and
C. The inability to demonstrate any other primary site. This tumour is characterized by aggressive and invasive behaviour which manifests as both local and distant metastases to sites such as lungs, liver, brain, and bones. Lymphatic metastasis at the time of diagnosis seems to be a crucial prognostic factor for oral melanomas. The American Joint Committee on Cancer (AJCC) does not have published guidelines for the staging of oral malignant melanomas; might be due to the rare occurrence of this lesion.
Westbury describes a clinical TNM classification for malignant melanomas as follows [1]:
a) Only primary tumour present.
b) Metastasis present
i. Adjacent skin involved,
ii. Regional lymph nodes involved, flab adjacent skin and regional lymph nodes involved.
c) Metastasis beyond regional lymph nodes.
Histologically, an oral melanoma is composed of sheets or islands of epithelioid melanocytes, which may be arranged in an organoid, or alveolar pattern. The cells have pale cytoplasm and large open nuclei with prominent nucleoli and occasionally they may be plasmacytoid. Sheets and fascicles of spindle cells may also be seen but are usually a minor part of the lesion. In most instances, the cells of melanoma contain melanin granules, but they may demonstrate no melanin production (amelanotic melanoma). Lack of production may cause diagnostic confusion at light microscopic level because melanoma can mimic a variety of undifferentiated tumours. Therefore, immunohistochemical studies are a guide to diagnosis. The immunoprofile of melanoma shows S100 positivity and negativity for cytokeratins in over 95% cases. Although sensitive, S100 is not specific. More specific markers include HMB45, Melan-A or anti-tyrosinase, which stain about 75% of lesions [1-5]. The morphological and immunohistochemical picture was on the same lines in our case also. Surgery remains the mainstay of treatment along with chemotherapy, radiotherapy, and immunotherapy. In the cases where metastasis has already occurred the disease is considered as classically incurable, surgery being considered only for palliative care and other treatment modalities like radiotherapy and chemotherapy taking precedence. Melanoma is notorious for its unpredictable and widespread metastasis. Metastatic spread to bone, usually the vertebrae, is a frequent finding in terminal disease and may be accompanied by multiple metastasis to the lymph nodes, central nervous system, lungs and liver. Metastasis to the oral regions is uncommon and usually involves soft tissues, notably the tongue.
The reported 5-year survival rate for oral malignant melanoma has ranged from4.5%to 29%withthemedian survival rate of 18.5 months after initial diagnosis. The median survival is affected by whether there is lymph node involvement (18 months) or not (46 months). Cutaneous melanomas can be graded by Clark levels or the Breslow tumour thickness grading system. The former classification assesses the depth of invasion, whereas Breslow’s system measures the thickness of the tumour from the surface of the epidermis to the greatest depth of the tumour. The risk for developing metastatic lesions from primary cutaneous melanomas increases with tumour thickness. The Breslow and Clark grading systems have not been validated as prognostic predictors in oral melanomas, probably owing to the rarity of this lesion. Additionally, in contrast to cutaneous melanomas, most oral melanomas are larger than 4 mm at the time of initial presentation. This factor, together with inadequate resection of margins and higher stage at initial diagnosis, may contribute to the discrepancy in patient’s 5-year survival rates between cutaneous melanoma (80%) and oral melanomas (15%). In general, the survival rates are poor and are worse for those with metastasis. Other factors associated with poor prognosis include, vascular invasion, necrosis, a polymorphous tumour cell population, and increasing age [2,4,6-8]. Malignant melanoma is a neoplasm that often tends to undergo regression. Clinically, variation in colour is perhaps the most important hallmark of primary cutaneous melanoma. The change in colour to white, off-white, blue-white and gray, white is a sign of (spontaneous) regression in malignant melanoma. Histopathologically the process starts with a dense lichenoid infiltrate of lymphocytes and ends with fibrosis and/or melanosis within a thickened papillary dermis. The dense infiltrate of lymphocytes permeates the thin melanoma and destroys the atypical melanocytes in the epidermis and the papillary dermis. The etiology of regression in melanocytic lesions is multifactorial, and the contributing mechanisms are yet to be completely elucidated. Literature search indicates that host-immune-mediated responses, particularly the CD8-positive cytotoxic T lymphocytes (CTLs) play a significant role in the development of regression. The role of the immune system in regression is further supported by the high incidence of CD4- positive T lymphocytes and Th1 cytokines in regressed tumours, as well as the high levels of tumour-specific antibodies and CTLs in the peripheral blood of patients with regressed tumours [8,9].
Conclusion
Early diagnosis and treatment are mandatory for better prognosis with regard to malignant melanoma of the oral cavity.
Clinicians must carefully examine the oral cavity and any growing pigmented lesion must be biopsied. Amelanotic melanomas can be diagnosed by immunohistochemical examination of tissue from the lesion. In addition, public education about self-examination of the oral cavity with periodic oral checkup is important for early detection of such lesions.
References
- Shirisha Rani G, Vinay Kumar T, Balaram Kolasani, Rezwana Begum, Anu Priya Srinivasan (2014) Primary Malignant Melanoma of Maxilla: Report of a Case with Discussion. Case Reports in Dentistry 2014: 1-4.
- Chaitanya YC, Chaitanya PV, Reddy MV, Bhavya B (2018) Oral malignant melanoma. J Indian Acad Oral Med Radiol 30: 158-160.
- Barnes L, Eveson JW, Recihart P, Sidransky (2005) World Health Organization Classification of tumours. Pathology and Gentics of Head and Neck Tumours. IARC Press, Lyon, France.
- Hashemi Pour MS (2008) Malignant melanoma of the oral cavity: A review of literatures. Indian J Dent Res 19: 47-51.
- Devi S, Sinha R, Singh RK (2015) Malignant melanoma maxilla. Natl J Maxillofac Surg 6(1): 115-118.
- Hasan S, Jamdar SF, Jangra J, Al Beaiji SM (2016) Oral malignant melanoma: An aggressive clinical entity - Report of a rare case with review of literature. J Int Soc Prev Community Dent 6(2): 176-181.
- Manigandan T, Sagar GV, Amudhan A, Hemalatha VT, Babu NA (2014) Oral malignant melanoma: A case report with review of literature. Contemp Clin Dent 5(3): 415-418.
- Ebenezer J (2006) Malignant melanoma of the oral cavity. Indian J Dent Res 17(2): 94-96.
- Gondivkar SM, Indurkar A, Degwekar S, Bhowate R (2009) Primary oral malignant melanoma--a case report and review of the literature. Quintessence Int 40(1): 41-46.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




