Lupine Publishers Group
Lupine Publishers
Review Article(ISSN: 2644-1209) 
Pupil Functions in Diabetes Mellitus Volume 1 - Issue 5
Ahmet OZER1* and Ayse Idil CAKMAK2
- 1 Department of Opthalomolgy, Eskisehir Osmangazi University, Turkey
- 2Department of Opthalomolgy, Hatay Mustafa Kemal University Tayfur Ata Sokmen, Turkey
Received: September 08, 2018; Published: September 18, 2018
*Corresponding author: Ahmet Ozer, Department of Ophthalmology, Eskisehir Osmangazi University Medical Faculty, Eskisehir, Turkey
DOI: 10.32474/TOOAJ.2018.01.000122
Abstract
Pupil is innervated by autonomic nerve system. As a result, its dimension or actions well reflects the autonomic nervous system function. Pupillary autonomic neuropathy is considered an early symptom of the development of systemic autonomic neuropathy. Pupil tests present suitable and easy methods for evaluation of autonomic nervous system function. Most patients with autonomic nervous system disorders show data of sympathetic or parasympathetic deficits in the pupil, and these findings can be detected using a combination of clinical signs, pupillometric and pharmacological tests.
Introduction
As a circular aperture in the middle of iris, along with leading aqueus humor from posterior chamber to anterior chamber, pupil has functions like regulating the amount of light entering the orbit, increasing the focusing depth, decreasing the spheric and chromatic aberrations. Under normal circumstances pupil has a diameter between 2-6mm with an average diameter of 3 mm. In neonatal period/babyhood pupilla has a smaller diameter that reachs its normal size at around 7-8 ages. On the other hand it is observed smaller in size in the old age, related on atrophy of iris stroma (1).
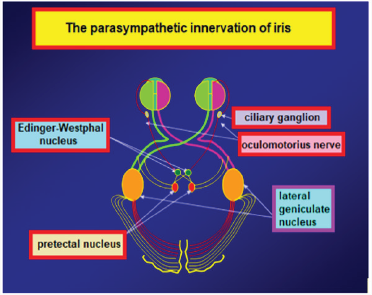
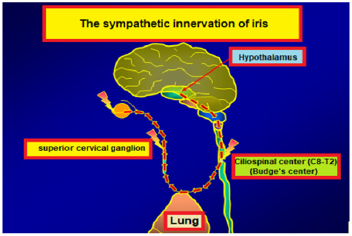
The muscles in the iris stroma are responsible miosis and mydriasis of pupil. These muscles are examined in two groups. The encircling muscle fibers all around the pupil are settled in the iris and they are named as circular and sphincter muscles innervated by parasympathetic system. When they contract miosis of pupil happens. The parasympathetic nerve fibers leading to miosis start from Edinger-Westphal nucleus and end in ciliary ganglion and nerve fibers that start from ciliary ganglion end in circular muscles (Figure 1).The other muscle fibers located perpendicular to the pupil are radial (dilatator) fibers. These fibers are sympathetically innervated and lead to mydriasis. The start point of the fibers that bring sympathetic impulses to the radial muscles is posterior hypothalamus. Nerve fibers originating from this region descend from mesensephalon and pons and end in the Budge ciliospinal center which is located in between 8th cervical and 2nd thoracic spinal segments of the medulla. 2nd nerve fibers for pupil which start from this region leave the medulla especially from 1st thoracic spinal segment. After being neighbor on apex of lung while ascending, these fibers join sympathetic chain at the level of inferior cervical ganglion. Fibers passing directly through the inferior and middle cervical ganglions end in upper cervical ganglion. 3rd nerve fibers starting from the upper cervical ganglion enter the cranium with internal carotid artery and communicates with ophthalmic branch of trigeminal nerve in the cavernous sinus. Sympathetic fibers reach the radial muscles, responsible for mydriasis, through the ciliary ganglion by the nasociliary nerve and the long ciliary nerves (Figures 1-2).
The tract of pupillary light reaction is composed of an afferent way that takes light impulse to the central nervous system, and an efferent way that takes the response to the iris muscles. This tract is composed of an arc containing four neurons. First neuron is in between retina and pretectal nucleus which is in the middle brain at the level of superior colliculus. Ganglion cells that start from retina end in this center. Light reaction starts with the excitation of retinal photoreseptors by light. Impulses, coming from temporal half of the retina by nerve fibers not crossing at the optic chiasma, reach the pretectal nucleus by passing through the optic tractus on the same side. However impulses coming from nasal half of the retina crosses in the chiasma and reach the pretectal nucleus by passing through the contralateral optic tractus. Nerve fibers responsible for light reaction leave the optic tractus and reach out the pretectal nucleus without a stop by lateral geniculate nucleus. The second neuron is the neuron that connects the pretectal nucleus, on one side, to both Edinger-Westphal nuclei. This neural tract is the reason of symmetric constriction of pupils at the same time when a light impulse is given. The third neuron starts with Edinger-Westphal nucleus. These parasympathetic nerve fibers interfere with the motor fibers that come from other nuclei of nervus occulomatorius and they reach the ciliary ganglion in orbita. The course of these fibers in the nervous occulomatorius is important. These superficial fibers that course between the brain stem and cavernous sinus are sensitive to pressure in this region. A lesion that presses on nervus occulomatorius in this region leads to 3rd cranial nerve palsy that also affects the pupil. The fibers related to pupil take part in the center of the nerve after the cavernous sinus. For this reason, even there are lesions in this area that cause external ophthalmoplegia, pupil fibers are preserved even. After the enterance of nervous occulomatorius into the orbita, the nerve fibers related to pupil leave the nerve and reach the ciliary ganglion within the inferior branch that goes to the inferior oblique muscle, while the fourth neuron starts from ciliary ganglion and ends in the circular muscles of iris. In orbita the ciliary ganglion is located in the extraocular muscle conus, just behind the eye ball. Only the nerve fibers that bring pupil’s light reaction form synapses in the ciliary ganglion, while the others don’t form any synapses while ending in their related places. Figure 1 Neural pathways related to accomodation start from retina like the fibers responsible for light reaction. Unlike the fibers related to light reaction, they reach the visual cortex by the route of chiasma, optic tractus and optic radiation. The source of the impulses that lead to accomodation is the peristriat cortex that is located in the 19th area on the superior end of calcarin fissure. The impulses that excite from here somehow activates the Edinger-Westphal nucleus. Fibers that start from Edinger-Westphal nucleus and come in orbita with nervus occulomatorus, reach the ciliary ganglion. A group of postganglionic nerve fibers that leave from here, goes to ciliary muscle for accomodation, another group goes to medial rectus muscle for convergence and another group goes to circular muscle of iris for miosis (1).
The examination of pupil reactions provides information about autonomic nerve system of the eye. In order to set out the functions of ocular autonomic nerve system, either pharmacological tests that show the denervation hypersensivity, or measurement methods like time of pupillary cycles that show the integrity of pupillary reflex arc can be used. In diabetes, there are some changes occur in pupil, as there are in all structures of the eye. In diabetic people, pupil is smaller than normal values, and its response to mydriatics is lower. The reason of pupil being smaller in diabetes is because of impairment of the balance between sympathetic and parasympathetic innervation. Being longer than the nerve trace of parasympathetic innervation makes the sympathetic innervation more vulnerable to development of sympathetic neuropathy. A neuropathy in sympathetic innervation causes the parasympathetic system become effective resulting in the pupil smaller in size. Failure in accomodation, can be seen in diabetes. Factors like infiltration of glycogen in the ciliary muscle, diabetic neuropathy or changes in lens structure can lead to failure in accomodation.
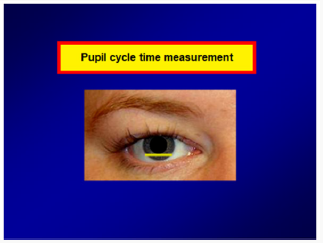
One of the methods that give some information about pupillary reflex arc is the measurement of pupillary cycle time. It practically gives information about the afferent and efferent ways of pupillary light reaction and the integrity of iris. This test is done by biomicroscope. The examination room is provided to be dark and the patient is waited at least two minutes to adapt to the darkness. The light of biomicroscope is set to be in horizontal position and 6x0.5 mm in size. The patient places his head on biomicroscope and he is looked at a small red light which is at least 5 metres away to avoid accomodation. Then the light of biomicroscope is slowly brought on the edge of the pupil to drop it on the retina. The pupil constricts by the effect of light. For this reason retina is exposed to darkness again and pupil tends to dilate again. Meanwhile again by the effect of light that is brought before, the pupil constricts and enlarges in a rhythmic process. When the rhythmic process starts, the time of 100 processes of constriction dilatation is determined by keeping a chronometer in milisecond (ms) type. The duration of this process can be determined both by a single time or by summing up 50+50 or 30+30+40 processes in order to get a total time of 100 constriction dilatation processes. While normal duration is between 850 ms and 950 ms, for applications done in specific clinics, it is better to determine the normal values of people in the same conditions who are in similar age groups (Figure 3).
It is shown that depending on the poor glisemic control of patients with diabetes melliltus, autonomic neuropathy can develop frequently and by affecting especially cardiovascular system vital complications can occur. At the same time it is found that symptoms of ocular autonomic neuropathy are the precursors of symptoms of systemic autonomic neuropathy. Pupil changes in diabetes can be grouped as below.
Pupil Changes in Diabetes
Myopathic causes: The storage of glycogen in iris muscles of people with diabetes has been shown in studies. There are two main muscle groups in iris as known. Being one of these muscle groups as mentioned before, circular muscle fibers that sorround the pupil are innervated by occulomotor nerve and responsible for miosis. The other muscle group is located perpendicularly to the pupil that is innervated by sypmpathetic nerve system and responsible for mydriasis. Accumulation of glycogen in iris muscles lead to contraction disfunctions. Histologically apart from accumulation of glycogen in iris muscles, vacuoles ares seen in the iris epithelium. These vacuoles are accepted as a sign of acute diabetes and thought of originating from abnormal storage of glycogen (2).
Neuropathic causes: Pupil changes developed by neuropathic causes, can be examined under three major headings.
Optic Neuropathy: Ischemic optic neuropathy are more seen in patients with diabetes. The form of pupil change in optic neuropathy is afferent pupillary defect. The direct pupillary light reaction is weaken or lost in the eye where optic neuropathy has developed. In the other eye it is the same subject for the indirect pupillary light reflex (3-5).
Cranial Neuropathy: Pupil changes can come out depending on affected occulomotor nerve that is one of the cranial nerves. In such a case there are findings like efferent pupillary defect. There are also findings added to the table which develop depending on affected motor fibers. Parasympathetic nerve fibers taking part in the third neuron start from Edinger-Westphal nucleus and extend to the ciliary ganglion that is located in the orbita. Because of the course of nerve fibers in the occulomotor nerve, any lesion located in a region between the brain stem and cavernous sinus and presses on occulomotor nerve, leads to 3rd nerve pasly which also affects pupil. On the other hand in the lesions locating after the cavernous sinus, pupillary fibers can be preserved. After the occulomotor nerve enters the orbita, the fibers that bring the pupillary light reflex reach the ciliary ganglion within the inferior branch that goes to inferior oblique muscle. In the occulomotor nerve palsy, superior, medial and inferior recti with inferior oblique muscles are affected. However usually because of ptosis developed by affected levator palpebralis superior muscle, patients don’t complain about diplopia. Usually pupil is not affected in diabetic occulomotor nerve palsy. Nevertheless it should be kept in mind that there are also diabetic palsies in which the pupil is affected.
Autonomic Neuropathy:
a) Sympathetic
b) Parasympathetic
In long lasting diabetes, a decrease in physiological hippus and a smaller pupil in size are detected. These effects are thought to result from selective diabetic autonomic neuropathy that rather shows itself significantly more in sympathetic system than parasympathetic system. Diabetic autonomic neuropathy which is a complication of diabetes, manifests itself as pupillary disfunction in the eye. Autonomic neuropathy is asymptomatic in the early stage and it can be reversible by glisemic control in the early stages of diabetes. In order to aviod permanent pathologies, scanning of autonomic disfunction is substantial. Pupil tests would be a method for the diagnosis of such a case at the earliest time. Pfeifer et al have shown that autonomic pupil disfunction has shown itself as miosis, and they has stated that this situation was due to impairment of balance between sympathetic and parasympathetic innervation, in favor of parasympathetic system (6).
Clark has determined that there are parasympathetic denervation supersensivity with %2,5 metacolin and sympathetic denervation supersensivity with %0,5 phenylephrin in patients with diabetic retinopathy. They had also determined a significant relationship between proliferative diabetic retinopathy and autonomic neuropathy. They had found that in proliferative cases, the incidence of rate of the parasympathetic retinopathy was %36, sympathetic neuropathy was %38; where as in nonproliferative cases they were %6 and %5 respectively (7).
Cahill et al have argued that the pupil responses to the %4 cocaine were same both in diabetics and control group, and for this reason they had argued that in both groups the sympathetic tract was intact (8). In the same study they had suggested that the changes in the superior cervikal ganglion were causing denervation hypersensivity in sympathetic tracts and this was the reason for the pupillary changes in diabetics. On the other side, (9) have studied the denervation sensivity of aproclonidin. They have shown the denervation sensivity in diabetic cases and found it to be proportionate to the duration and severity of the disease (5).
Söylev et al. (10) have studied the response to diluted pilocarpine in groups of diabetic patients with or without retinopathy and in control group. They have found a response to diluted pilocarpine more in diabetic group with retinopathy, according to diabetic group without retinopathy and control group (10). At the same time they have detected that marked mydriasis developed with diluted phenylephrine in diabetic group with retinopathy, which was found significantly different comparing to the other groups.
Clark et al. (11) have detected the rate of the defect in pupillary reflex arc as %88,5 in patients with proliferative diabetic retinopathy. They have also mentioned that this result was the earliest sign of autonomic nerve disfunction (11). Pena et al. (12) emphasized that the pupillary symptoms were the earliest evidence of autonomic nerve disfunction.
In our study the pupil cycle time was found longer in diabetic cases. At the same time the rate of inability of measuring pupil cycle time was found more in the diabetic group. Pupil cycle time was found related both with the existence of diabetic retinopathy and the level of periferic retinopathy (13). As seen in all structures of eye, there are some changes in diabetes that can be seen in the pupil, too. These changes can be basicly myogenic or neurogenic. As a complication of diabetes, diabetic autonomic neuropathy can show itself as pupillary disfunction in the eye, so early diagnosis is significant. Pupil findings usually come out in direct proportion to the length and severity of diabetes (14). Rarely they can be seen in the early stages of diabetes; even sometimes before the somatic neuropathy. The findings of cardiovascular system among the systemic autonomic neuropathy findings are vitally important. By determining the abnormalities of pupillary function earlier, growth of autonomic function abnormalities of cardiovascular system may be prevented and measures for diabetic regulation may be taken. Autonomic neuropathy is asymptomatic in early phase and it can be reversible by glisemic control in early stages. From this point, scanning for autonomic disfunction is important to prevent permanent pathologies. Pupil tests can be methods for diagnosis of this situation in the earliest time.
References
- Miller NR, Newman NJ (1999) The Essentials: Walsh and Hoyt’s Clinical Neuro-ophthalmology (5th edn.); Williams and Wilkins Baltimore.
- L’Esperance FA, James WA (1983) The eye and diabetes mellitus. In Ellenberg M, Rifkin H (Eds.); Diabetes Mellitus: Theory and Practice (3rd edn.); Medical Examination Publishing, New York, USA, pp. 727-758.
- Lee MS, Grossman D, Arnold AC (2011) Incidence of nonarteritic anterior ischemic optic neuropathy: Increased risk among diabetic patients. Ophthalmology 118(5): 959-963.
- Hayreh SS, Zimmerman MB (2008) Nonarteritic anterior ischemic optic neuropathy: Clinical characteristics in diabetic patients versus nondiabetic patients. Ophthalmology 115(10): 1818-1825.
- Hayreh SS, Joos KM, Podhajsky PA, Long CR (1994) Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 118(6): 766-780.
- Pfeifer MA, Cook D, Brodsky J, Tice D, Parrish D, et al. (1982) Quantitative evaluation of sympathetic andparasympathetic control of iris function. Diabetes Care 5(5): 518-521.
- Clark CV (1988) Ocular autonomic nerve function in proliferative diabetic retinopaty. Eye 2: 96-101.
- Cahill M, Eustace P, de Jesus V (2001) Pupillary autonomic denervation with increasing duration of diabetes mellitus. Br J Ophthalmol 85(10): 1225-1230.
- Koc F, Kansu T, Kavuncu S, Firat E (2006) Topical apraclonidine testing discloses pupillary sympathetic denervation indiabetic patients. J Neuroophthalmol 26(1): 25-29.
- Söylev M, Saatçi A, Durak İ (1996) Diabetes Mellitusta Pupilla Disfonksiyon. T Klin J Oftalmoloji 5: 114-116.
- Clark CV, Ewing DJ (1988) Ocular autonomic function in progressive autonomic failure. Doc Ophthalmol 70(4): 309-321.
- Pena MM, Donaghue KC, Fung AT, Bonney M, Schwingshandl J, et al. (1995) The prospective assessment of autonomic nerve function by pupillometry inadolescents with type 1 diabetes mellitus. Diabet Med 12(10): 868-873.
- Efe B, Kutlu C, Özer A, ve ark (1992) Diabetik Nöropati. Ulusal Endokrinoloji Dergisi 2(1): 77-94.
- Özer A (2014) Diyabet ve Pupilla, Ret Vit Özel Sayı 22: 179-184.