Lupine Publishers Group
Lupine Publishers
Review ArticleOpen Access
Ophthalmic Manifestations of COVID-19 and its Association with Heparan Sulfate Receptors Volume 3 - Issue 3
Shyam Sundar Nandi1, Ilina Bhattacharya2, Upendra Lambe1, Sonali Sawant1, Trupti Gohil1, Tejabhiram Yadavalli2, and Deepak Shukla3*
- 1National Institute of Virology, Mumbai Unit, A.D. Marg, Parel. Mumbai, India
- 2Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago, Chicago, USA
- 3Department of Microbiology and Immunology, University of Illinois at Chicago, Chicago, USA
Received:May 11, 2021; Published:May 28, 2021
Corresponding author: Deepak Shukla, Department of Microbiology and Immunology, University of Illinois at Chicago, Chicago, IL 60612, USA
DOI: 10.32474/TOOAJ.2021.03.000161
Abstract
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
Coronavirus disease-2019 (COVID-19) caused by a novel enveloped, positive sense, single stranded RNA virus called Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) started originally in Wuhan, China. By March 2020, the disease was declared a global pandemic as the virus spread to all major countries in the world. Initially, it was believed that the virus was transmitted through inhalation of respiratory droplets from an infected person. But given the exponential increase in the number of infected people, more modes of transmission were explored. While all possible routes of transmission of this virus are still undetermined many studies implicate the eyes as the initial site of infection and conjunctivitis as an early symptom of COVID-19. In this review, we summarize various studies that suggest SARS-CoV-2’s presence on ocular surfaces and that the eyes can be a gateway for transfer of SARS-CoV-2 to the extraocular sites including the lungs. We also explore the role of heparan sulfate, a newly discovered co-receptor for the virus in ocular manifestations.
Keywords: SARS-CoV-2; COVID-19; Ocular; Heparan Sulfate; ACE 2; Conjunctivitis
Introduction
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
Coronaviruses (CoV) are a large family of enveloped RNA viruses. Structurally, a typical virion consists of envelope proteins and spike proteins protruding from its envelope surrounding the nucleocapsid. The spike proteins play an important role in binding to host cells. These viruses cause severe respiratory and enteric infections in humans as well as animals. A newly discovered strain, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is responsible for the ongoing global outbreak of coronavirus disease 2019 (COVID‐19) [1]. Initially pneumonia, cough and respiratory problems were the only reported symptoms for COVID-19 [2]. But there have been many reports of inflammation and irritation of eye in COVID-19 patients. While conjunctivitis or pink eye has not been established as an official symptom for COVID-19, few studies indicate it as an ocular manifestation of SARS-CoV-2 infection [3, 4]. To provide some history, a study conducted during the 2003 Severe Acute Respiratory Syndrome (SARS) outbreak detected SARS-CoV in tear samples of SARS patients in Singapore [5]. Insufficient eye protective equipment was considered to be one of the reasons for SARS-CoV transmission, indicating a concern that respiratory illness could be transmitted through ocular secretions [6,7]. Similar alarming situations have been raised with SARS-CoV-2 especially amongst the healthcare professionals involved with eye care and the healthcare workers present in the triage area and involved with checking symptoms of the patients and sample collection [8]. This also puts ophthalmologists and other healthcare workers at risk who are examining the COVID-19 patients manifesting conjunctivitis. An Ophthalmologist, Li Wenliang, MD died because of COVID-19. The source of transmission of the virus was later found out to be an asymptomatic glaucoma patient who visited his clinic [8]. COVID-19 is normally believed to be transmitted by respiratory droplets [9]. However, some growing body of evidence links conjunctivitis to the early stages of COVID-19 infection [4]. The ocular surface might also act as a point of entry and facilitate coronavirus transmission. Thus, to combat a global threat like COVID-19 with many asymptomatic patients, it is imperative to understand these other unexplored pathways for infection and the underlying mechanisms.
SARS CoV-2 Host Cell Entry
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
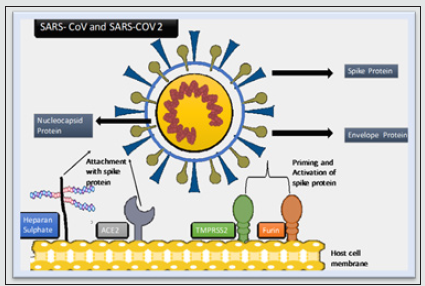
SARS-CoV-2 entry into the host cell is mediated via its spike glycoprotein S. The spike uses angiotensin-converting enzyme 2 (ACE2) as its receptor on host cells to facilitate the infection as a primary receptor [10]. SARS-CoV-2 spike protein binds with both cell surface heparan sulfate receptor and angiotensin-converting enzyme 2 (ACE2) by binding with its receptor-binding domain (RBD). Docking studies suggest a heparin/heparan sulfate-binding site adjacent to the ACE2-binding site. Both ACE2 and heparin can bind independently to spike protein in vitro, and a ternary complex can be generated using heparin as a scaffold. Electron micrographs of spike protein suggest that heparin enhances the open conformation of the RBD that binds ACE2. On cells, spike protein binding depends on both heparan sulfate and ACE2 [11]. In a study conducted by Wan and co-workers, it has been shown that the S glycoprotein of SARS-CoV-2 has a receptor binding domain (RBD) and the residue 394 (Glutamine) is responsible for binding with ACE2 receptors [12]. The S glycoprotein is activated by proteolytic cleavage by transmembrane serine protease (TMPRSS2) or the protease Furin (also known as Paired Basic Amino Acid Cleaving Enzyme) for interaction with ACE2 [13]. In TMPRSS2-negative cells, the cysteine proteases cathepsin B/L can facilitate S protein cleavage [14]. Furin acts by cleaving S1 subunit of spike protein which leads to conformational changes in S2 subunit of the spike protein. These changes expose the membrane proteins needed for virus to fuse with membrane to enter the cell as represented in Figure 1[13]. Certain human coronaviruses like HCoV-NL63 need co-receptor such as heparan sulphate (HS) in addition to ACE on host cell membrane for the virus to bind and facilitate its entry into the host cell. HS has also been indicated to play an important role in SARS-CoV’s ability to infect [15]. Studies show reduction in heparin or heparinase leads to decrease in SARS-CoV entry in cells [16]. SARS-CoV-2 displays conformational changes during binding of the virus RBD and the host cell heparan sulphate [17].
Figure 1: SARS-CoV and SARS-CoV 2 viruses enter the host cell by interacting with ACE 2 and heparan sulfate. Cell surface protease TMPRSS2 and Furin activates the SARS-CoV virus entry.
Ocular Viral Transmission
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
Globally, governments are trying to impose different preventative measures to contain COVID-19. All nations are employing nationwide lockdowns, but epidemiologic data indicate differences in disease incidence. Some countries have been successful in flattening the curve whereas even after stay at home orders, the cases are exponentially rising in some other countries. This points towards an incomplete understanding of the modes of transmission. Several studies suggest transmission modes through aerosols and fomites especially given the high transmissibility rate and some molecular characteristics of the virus [18]. Thus, to reduce disease transmission, it is necessary to research into all possible ways of disease transmission.
Previous studies have demonstrated that the mucosa of the ocular surface and respiratory tract express identical receptors for certain respiratory viruses [19-22]. On the basis of the epidemiological information from earlier Coronavirus infections, various theories have been anticipated such as:
a) The conjunctiva can act as a site of direct inoculation by droplets containing virus particles.
b) The nasolacrimal duct acts as a route of virus infection to the upper respiratory tract.
c) Haematogenic (from blood) infection of the lacrimal glands [23].
In case of SARS-CoV-2, also a respiratory virus, interaction of its spike protein with host ACE2 is responsible for viral entry as well as human-to-human transmission [9,10]. Thus, the expression of receptor ACE2 on the surface of corneal epithelium and conjunctival epithelial tissues indicates a plausible role of eye in COVID-19 transmission. But the level of ACE2 expression observed in the ocular tissues was found to be much lower than the respiratory and kidney tissues [17]. Also, the binding ability of SARS-CoV spike protein to the ACE2 expressed on the ocular surfaces was observed to be weaker than the binding ability with the ACE2 receptors on the surface of Vero E6 cells in-vitro and the lung tissues in-vivo [20].
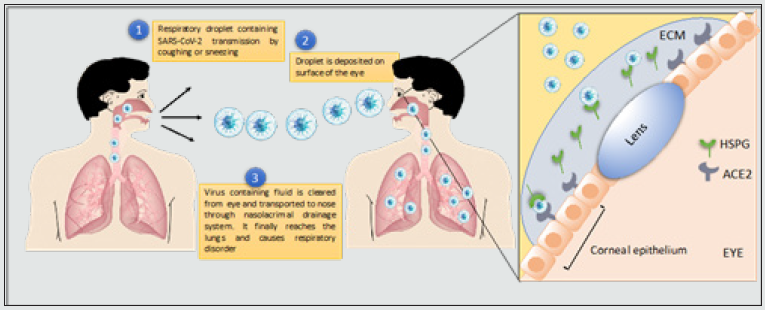
In this review, we suggest ocular transmission in addition to transmission by respiratory droplets, fomites and aerosols as a mode of SARS-CoV-2 transmission. Respiratory viral infections leading to development of ocular symptoms have been previously documented [4]. Scientists have hypothesized a model for eye as a gateway to transmission of virus to the respiratory tract. According to the anatomy, the mucosa of the conjunctiva and corneal epithelium and the upper respiratory tract are connected by the nasolacrimal duct [19]. When a drop of liquid is inserted into the eye the liquid is partially absorbed by the cornea and conjunctiva but mostly is passed into the nasal cavity through nasolacrimal duct and then transported to the upper respiratory tract including pharynx and trachea or else it can be taken to the gastrointestinal tract as shown in Figure 2 [24]. This ocular surface to systemic transmission hypothesis originally proposed by Belser has been further corroborated by viral inoculations of adenoviruses and influenza viruses in the cornea of animal models including mice, rats and rabbits. And presence of viral loads in tear samples from these animals have been detected [19]. CoVs can result in a wide spectrum of ocular infections in animals. The conjunctival swabs of 90% cats infected with feline CoV (FCoV) had the FCoV antigen. This indicates the probability of ocular manifestations of SARSCoV- 2 in patients similar to the CoVs of animals [25]. The potential of infection through ocular secretions is currently unknown, and it remains unclear how SARS-CoV-2 accumulates in ocular secretions. Possible theories include direct inoculation of the ocular tissues from respiratory droplets or aerosolized viral particles, migration from the nasopharynx via the nasolacrimal duct, or even hematogenous spread through the lacrimal gland [26].
Figure 2: Transfer of SARS-CoV 2 from the eyes to the lungs occurs via nasolacrimal system. Respiratory droplets containing SARS virus weakly attach to HS and ACE 2 on the epithelial lining of the cornea. Due to lower affinity interactions with its receptors on the corneal surface, the virus, in most cases, fails to enter the ocular cells and it is released in the tear fluid, which is then absorbed by the lacrimal duct and the virus is transported to the nose and then the lungs
Conjunctivitis and Its Correlation with Coronavirus
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
Conjunctivitis (pink eye) is inflammation of the transparent membrane that lines the eyelid (conjunctiva) due to infections or allergies. The symptoms are watery, itchy red eyes with crusting around the eyes [27]. Many viruses can cause conjunctivitis. Unlike antibiotic eye drops for conjunctivitis caused by bacteria, viral conjunctivitis is self-healing [4]. Viral conjunctivitis is caused by DNA viruses like adenoviruses or herpesviruses and RNA viruses like picornavirus and HIV. Thus, conjunctivitis is a common ocular manifestation observed with many viruses [4]. During previous coronavirus outbreaks like SARS-CoV and HCoV-NL63, Real-time Reverse transcription polymerase chain reaction (rRT-PCR) in tears have detected the presence of virus [28, 29]. But many reports for SARS‐CoV or MERS‐CoV do not recognize conjunctivitis as a sign or symptom [30]. For NL63 infections, a review combined 15 studies with almost 7,000,000 patients and conjunctivitis was reported in only 17 % of one study of 300 patients [15]. Additionally, some strains of coronavirus infections displayed no viruses in their tears. Considering the history of viruses and many human case studies for SARS-CoV-2, there is a growing interest among researchers to study ocular manifestation of coronaviruses.
Animal studies
Animal studies can help to better understand the effects of coronavirus on eye after infecting the eye with coronavirus.
Animal models and infection techniques to study specifically ocular manifestation of SARS-CoV has not been established yet. Studies for other types of coronaviruses, however, have been established. There are severe ocular manifestations due to coronaviruses in cats and mice like anterior uveitis, retinitis, vasculitis, and optic neuritis [25]. One non-peer reviewed article compared the effects of SARSCoV- 2 infection in different anatomical sites in rhesus monkey – eye (conjunctiva), lungs (trachea) and stomach. Monkeys that were only injected via trachea experienced weight loss. After 24 hours of infection, viral RNA was detected only in conjunctival swabs of monkeys infected via the eyes. These monkeys also had mild lung infection. For the infection via trachea, there was presence of SARSCoV- 2 RNA in the nasal and throat swabs of the monkey [31]. This animal study sheds light on the significance of site of manifestation of infection being related to the transmission route of the infection. Thus, using experimental designs from previous coronavirus animal models, more studies need to be conducted to understand ocular transmission and manifestation of SAR-CoV-2. Histology studies should be performed on infected eye to completely understand these ocular manifestations.
Standardization of techniques needed for detection of viral conjunctivitis
It has been observed that viral conjunctivitis is caused by direct inoculation of the virus in the conjunctiva for animal models. Different COVID-19 patients seem to have variations in the conjunctivitis manifestations. One patient with COVID-19 had viral RNA in conjunctival swabs taken just after two days of infection, whereas another patient displayed bilateral conjunctivitis for 21 days after the infection. Another case study report had a COVID-19 patient with conjunctivitis but with no viral RNA in tears [32]. These studies show the diversification and differences in these ocular manifestations patient to patient. Thus, more sensitive techniques need to be adapted to track virus induced conjunctivitis for more accurate data. Different instruments have been used to analyze or detect viral RNA in patients. One study collected tear using a Schirmer strip and analyzed viral RNA in tears by rRT-PCR. They collected samples for 3 weeks from the onset of infection [26]. In this study, one patient had conjunctivitis and viral RNA was detected in tears. Another study used swab to collect conjunctival secretions from lower fornix of the eye [33]. They also collected tear data from the lower fornix. In some other studies, tear samples were collected from lower fornix and conjunctival scraping from lower palpebral conjunctiva. The eyelids were everted and then the lower fornixes were swiped with sterile cotton swabs to collect the samples. No topical anesthesia was given. Another study collected tears randomly on nine days and 19.4 days after infection [5]. While three positive cases were found in the 9-day cohort, negative results were observed in 19.4 days sample set. The available data shows very low number of cases having ocular manifestations of COVID-19. Additionally, the tear or conjunctival swab samples tested positive for SARS-CoV-2 in a single study was not significant. Therefore, a more standardized method for detection of SARSCoV- 2 in tear samples is required. By testing of large number of samples a data can be generated to provide better understanding of ocular manifestation of COVID-19. Masaki Imai et al., suggest that Syrian hamsters are susceptible to SARS-CoV-2 replicating efficiently in lungs and causing severe pathological lesions [34]. They also reported that SARS-CoV-2 can replicate in the brain or olfactory bulb of hamsters but failed to detect viral antigens in these regions. Chan et al. also demonstrated use of Syrian hamster model for SARS-CoV-2 transmission studies [35].
Human case studies
In case of the ongoing pandemic COVID-19, there have been multiple reports of conjunctivitis during initial stages of the infection. Recently, a woman with COVID-19 was reported to have unilateral conjunctivitis with other symptoms like cough and nasal congestion. Her initial diagnosis was presumed to be herpetic keratoconjunctivitis. Antiviral treatment for herpes had no effect in reducing the symptoms and the unilateral conjunctivitis remained for seven days. She was tested positive for SARS-CoV-2 after seven days. The laboratory diagnosis revealed the patient was weakly positive for SARS-CoV-2 and was found negative for other secondary bacterial infections [36]. There was another similar case where the patient initially diagnosed with conjunctivitis, reported fever symptoms two days later. On testing, the patient was found to be SARS-CoV-2 positive [37]. On January 22, 2020, a Chinese respiratory specialist who visited Wuhan as a member of the national expert panel on pneumonia claimed that he was infected by SARS-CoV-2 despite being fully gowned with a protective suit and N95 respirator. His first clinical manifestation was unilateral conjunctivitis, followed by fever and catarrhal symptoms 2 or 3 h later. It was postulated that SARS-CoV-2 probably first infected the conjunctiva, then spread and caused viral pneumonia [38]. However, in an another study by Zhou and colleagues, it was reported that conjunctivitis was identified only in one patient out of 63 COVID-19 cases and 4 suspected COVID-19 cases. Conjunctivitis was also the first symptom of SARS-CoV-2 infection in this patient. However, RT-PCR in conjunctival swab samples was positive for SARS-CoV-2 RNA from only one COVID-19 patient without conjunctivitis. This patient had no ocular symptoms. SARS-CoV-2 RNA was not detected in conjunctival swab in another similar case study of an anesthesiologist with COVID-19 and conjunctivitis. Her ocular symptoms occurred soon after performing tracheal intubation for a patient who was confirmed as having COVID-19 later, and this was followed by fever and cough. Unfortunately, the personal protections used by this anesthesiologist during the tracheal intubation procedures were only a surgical mask, cap, and gloves, without a gown, face shield or goggles. Her five colleagues were also infected by the same patient, yet none of them exhibited any ocular complications [39]. Zhang and colleagues, reported conjunctivitis in two patients out of 72 laboratory-confirmed COVID-19 cases; however, SARS-CoV-2 was detected in conjunctival swab samples by rRT-PCR in only one patient who was a nurse working in the Emergency Department. This patient presented with excessive tearing and redness in both eyes, which were typical ocular manifestations of viral conjunctivitis, accompanied by a moderate fever of 38.2 °C that occurred 1 day earlier. SARS-CoV-2 rRT-PCR tests for the conjunctival and oropharyngeal swabs sampled 2 days after the onset of fever was positive, but for those sampled 9, 18, and 20 days after the onset of fever were all negative [40]. Xia and colleagues reported unilateral conjunctivitis in one patient out of 30 confirmed COVID-19 cases; conjunctival swabs sampled from this patient 3 and 5 days after the onset of COVID-19 were both positive for SARS-CoV-2 by rRT-PCR, whereas 58 conjunctival swab samples from the other 29 COVID-19 patients were all negative for SARS-CoV-2. However, SARS-CoV-2 was not isolated and cultured in the conjunctival swab samples from the COVID-19 patient with conjunctivitis. In contrast, 55 of the 60 sputum samples from 30 COVID-19 cases showed positive rRT-PCR results for SARS-CoV-2 [41].
Meta analytical studies
Although, ocular manifestations of COVID-19 in form of conjunctivitis are overall rare in the published literature. Only 9 (0.8%) out of 1,099 patients from 552 hospitals across 30 provinces in China were reported to have “conjunctival congestion.” [42]. A recent case series reported ocular symptoms in 12 (31.6%) of 38 hospitalized patients with COVID-19 in Hubei province, China [43]. These patients had conjunctival hyperemia (3 patients), chemosis (7 patients), epiphora (7 patients), or increased secretions (7 patients). Of note is that one patient who had epiphora presented with epiphora as the first symptom of COVID-19. Of those with ocular manifestations, 2 (16.7%) patients had positive results of SARS-CoV-2 on rRT-PCR by a conjunctival swab as well as by nasopharyngeal swabs. Only one patient in this study presented with conjunctivitis as the first symptom. The authors noted that patients with ocular symptoms had higher white blood cell and neutrophil counts, C-reactive protein, and higher levels of procalcitonin and lactate dehydrogenase compared to patients without ocular abnormalities [43]. Similarly, in an another cross sectional study, out of 535 patients, 27 patients (5.0%) showed congestion in conjunctiva and 4 patients showed conjunctival congestion as the early symptom. The mean time period of conjunctival congestion was 5.9 ± 4.5 days [SD]. The other ocular symptoms, including increased conjunctival secretion, ocular pain, photophobia, dry eye and tearing, were also found in patients with conjunctival congestion. Notably, hand–eye contact was independently correlated with conjunctival congestion in COVID‐19 patients. We also found that some COVID‐19 patients had chronic eye diseases, including conjunctivitis (33, 6.2%), xerophthalmia (24, 4.5%) and keratitis (14, 2.6%). Similar to the published studies, the most common clinical symptoms were fever, cough and fatigue. A total of 343 patients (64.1%) had positive SARS‐CoV‐2 detection in nasopharyngeal swabs [44].
In a comparison of the interaction between the animal-tohuman transmitted coronaviruses (SARS-CoV-1, SARS-CoV-2, MERS-CoV, CoV-229E, NL63, OC43, HKU1) and the eye by Sharif et al., the limit for detection of viral RNA in ocular discharge was 0–8% for SARS-CoV-1 and 0–5.3% for SARS-CoV-2, while no reports were found for other coronaviruses. Ocular manifestations have been encountered for NL63 and SARS-CoV-2. Ocular clinical symptoms in the form of conjunctivitis/conjunctival congestion were mainly detected in 65 (3.17%) out of 2048 reported patients with COVID-19 (range of 0.8–32%). Eye symptoms were not reported for the other coronaviruses [45].
On 20th Mar 2020, Sarma, et al. screened 5 different literature databases (PubMed, Google Scholar, EMBASE, Medrixv, and BioRixv). In their systematic review and meta-Analysis, authors included studies about the ocular manifestation of SARS-COV-2 patients were without language restriction. This study concluded that 3.17% of patients show ocular manifestation. However, only 1.949% of patients show tear/conjunctival swab RT-PCR positivity. However, in spite of presence of the virus in the ocular fluid, only 33.3% showed sign of conjunctivitis/conjunctival chemosis or red eye. Again vice versa, i.e. among patients with COVID-19 associated conjunctivitis/red eye, only 28.65% showed evidence of presence of the virus in ocular fluid [46]. Loffredo et al. evaluated the frequency of conjunctivitis in patients affected by severe and non-severe COVID-19 infection according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) only on clinical studies identified by searching Pubmed, ISI Web of Science, SCOPUS, and Cochrane electronic databases. On 5th Apr 2020, authors included 1,167 COVID-19 patients in their metaanalysis. The rate of conjunctivitis was 1.1%; it was 3% and 0.7% in severe and nonsevere COVID‐19 patients, respectively. The metaanalysis showed that patients with severe COVID‐19 infection had, at admission to the hospital, increased incidence of conjunctivitis (odds ratio: 3.4; 95% confidence interval:1.1‐10.2; P = .030) [47].
Siedlecki, et al. used the PubMed.gov for searching relevant articles. On 16th Apr 2020, authors identified 21 articles on the ophthalmological aspects of COVID-19 were identified. Of these, 12 (57.1%) were from Asia, 6 (28.6%) were from the United States of America, and 3 (14.3%) were from Europe. There were 5 (23.8%) original studies, 10 (47.6%) letters, 3 (14.2%) case reports, and 3 (14.2%) reviews [48]. On 29th May 2020, Emparan et al. published a structured review on COVID-19 and ophthalmology using PubMed, ScienceDirect, LILACS, SciELO, the Cochrane Library, and Google Scholar as electronic databases. The Oxford Center for Evidence- Based Medicine 2011 Levels of Evidence worksheet was employed by authors for quality assessments. More than 1,000 manuscripts were identified in the research; only 26 records were included in the qualitative synthesis and of these only 17 were classified as level 5 within the classification system of the Oxford CBME methodology, the rest were level 4 [49]. Lastly, on 16th Jun 2020, Torres-Costa et al. reviewed the most relevant articles together with the official recommendations of ophthalmological societies by literature search on PubMed electronic database [50]. Investigations have revealed that highly infectious human CoVs (mainly SARS-CoV and SARS-CoV-2) are rarely detected by rRT-PCR and never isolated by virus culture in tears and conjunctival secretions from SARS and COVID-19 patients. Hence, it is hard to assess the infectivity of tears and conjunctival secretions and their roles in virus transmission [38-51].
Receptors Involved in Ocular Manifestations
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
It has been observed that SARS-CoV-2 : has a stronger binding affinity towards ACE2 in comparison to SARS-CoV due to differences in the spike protein gene. ACE2 is the receptor required for SARS-CoV-2 entry into the host and is expressed in conjunctival epithelial cells and corneal cells and tissues [52]. Although expression of ACE2 in eye is much less than that in heart, lung tissues and Vero E6 cells. In cornea, expression of mRNA for ACE2 has also been reported. This mRNA expression in cornea is lower in comparison to testes, small intestine and heart [52]. As discussed TMPRSS2 or Furin which are required to activate spike protein are not found in corneal epithelium or conjunctival cells. The average mRNA expression for TMPRSS2 in these corneal cells is around 0.6 % which is usually in the range 1.2-1.4 % in tissues like lung parenchyma, heart and nasal cavity [52]. In a study conducted by Zhou and coworkers, various eye specimens were tested for expression of ACE2. Across all eye specimens, immunohistochemical analysis revealed expression of ACE2 in the conjunctiva, limbus, and cornea, with especially prominent staining in the superficial conjunctival and corneal epithelial surface. Surgical conjunctival specimens also showed expression of ACE2 in the conjunctival epithelium, especially prominent in the superficial epithelium, as well as weak or focal expression in the substantia propria. All eye and conjunctival specimens also expressed TMPRSS2. These results suggest that ocular surface cells including conjunctiva are susceptible to infection by SARS-CoV-2, and could therefore serve as a portal of entry as well as a reservoir for person-to-person transmission of this virus. This highlights the importance of safety practices including face masks and ocular contact precautions in preventing the spread of COVID-19 disease [39]. On the other hand, in a study conducted by Ma and coworkers, rRT-PCR analysis showed consistent expression by 2 ACE2 gene primers in 2 out of 3 human conjunctival cells and pterygium cell lines. Expression by 2 TMPRSS2 gene primers could only be found in 1 out of 3 pterygium cell lines, but not in any conjunctival cells [53]. Compared with the lung A549 cells, similar expression was noted in conjunctival and pterygium cells. In addition, mouse cornea had comparable expression of TMPRSS2 gene and lower but prominent ACE2 gene expression compared with the lung tissue. Considering the necessity of both ACE2 and TMPRSS2 for SARSCoV- 2 infection, the results suggest that conjunctiva would be less likely to be infected by SARS-CoV-2, whereas pterygium possesses some possibility of SARS-CoV-2 infection. The cornea has shown higher level of expression of ACE2 and TMPRSS2 than conjunctiva. Hence, the cornea has higher potential to be infected by SARSCoV- 2 [53].
Other receptors expressed in human corneal and conjunctival cells that can bind to SARS-CoV-2 are CD209 (on corneal dendritic cells) and CD26 (vascular endothelial cells), CD13, 9‐O‐acetylated sialic acid and heparan sulfate (HS). These receptors usually found in human tears act as binding site for coronaviruses. SARS-CoV binds to CD209 and CD26 [32, 54]. Human CoV-229E binds to CD13 for cell infection. HKU-1 binds to 9‐O‐acetylated sialic acid [55]. NL63 binds to ACE-2 by initial attachment to HS before binding to ACE2 [15]. But this binding alone does not cause an infection. HS has been previously indicated to help in viral attachment in addition to ACE2 for many viruses. But the presence of lactoferrin on ocular surface can prevent this binding to HS. This data suggest that coronaviruses might weakly bind to ocular surfaces but may not necessarily cause an infection especially due to lack of proteases TMPRSS2 or Furin [32].
Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
The receptors intercellular adhesion molecules – 1 (ICAM-1) or αvβ3 and αvβ6 integrin facilitate conformational changes in virus after the initial binding during viral conjunctivitis. The other host receptors low density lipoprotein (LDL) and heparan sulfate proteoglycan (HSPG) also aid in viral endocytosis. For many other viruses HSPG is known to enhance viral binding [4]. Studies have revealed that the invasion of SARS-CoV and HCoV-NL63 into host cells not only relies on the presence of ACE2 on host cell membrane as an entry receptor but also is modulated by other factors on host cell membranes such as HSPG, which serves as an attachment receptor [15, 56].
Heparan Sulfate
HS is a complex carbohydrate. It has sulfate residues attached at oxygen and nitrogen sites [57]. HS is ubiquitously expressed on the surfaces and in the extracellular matrix of virtually all cell types, making it an ideal receptor for viral infection [58]. It occurs in the form of proteoglycan HSPG in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix protein [59,60]. A complex biosynthetic process is responsible for the production and modification of HS, which takes place through coordinated action of several glycosyltransferases and sulfotransferases. HS is composed of repeating disaccharide units of glucosamine and uronic acid, with variable additions of sulfate groups and other modifications. As a result of its high sulfation, HS has an extreme negative charge density, and thereby binds with a large variety of extracellular ligands, including growth factors, cytokines, and enzymes [61]. HS has been known for many years to serve as a major attachment receptor for many human viruses, including dengue virus, hepatitis C virus, human immunodeficiency virus, human papilloma virus, and essentially all herpes viruses [62-64]. In this form, the HS binds to a variety of protein ligand and help in the regulation of various biological functions including developmental process, angiogenesis (Formation of new blood vessels), coagulation of blood, antagonists for Granzyme B and also acts as receptor for various viral infections including SARS-CoV-2 [65-66]. HS is known for interacting with different proteins to regulate and facilitate cell adhesion, the cell cycle, and inflammation. As previously mentioned, many viruses need HS as a co-receptor for entry into the host [57].
Role in virus attachment
To demonstrate the interaction between SARS-CoV-2 spike protein and HS receptor, a study was conducted by Liu and coworkers [17]. In this study, a HS oligosaccharide library showed the spike of SARS-CoV-2 can bind HS in a length- and sequence-dependent manner. A controlled HS synthesis was used for production of attached sulfate residues in different lengths with varied patterns and numbers. It is believed that different cells express specific HS binding sites to attract the HS specifically needed to regulate a metabolic process. In many other proteins, this preferential binding to certain repeating pattern of oligosaccharides has been observed. Liu and coworkers screened a different HS library with varying and different patterned chains and studied viral binding to each specific HS molecule. They also used cell culture and drosophila model to study spike protein and RBD binding of the virus respectively. They have identified two types of HS molecules having greater affinity towards spike protein of SARS-CoV-2. Hexaand octasaccharides composed of IdoA2S-GlcNS6S repeating units were identified as optimal ligands. Surface plasma Surface Plasmon Resonance (SPR) showed the SARS-CoV-2 spike protein binds with higher affinity to heparin (KD 55 nM) compared to the RBD (KD 1 μM) alone [17]. An octa-saccharide composed of IdoA2S-GlcNS6S could inhibit spike-heparin interaction with an IC50 of 38 nM. The data supports a model in which the RBD of the spike of SARS-CoV-2 confers sequence specificity for HS expressed by target cells [17]. An additional HS binding site in the S1/S2 proteolytic cleavage site enhances the avidity of binding. Glycosaminoglycan (GAG) binding motif at S1/S2 proteolytic cleavage site on spike glycoprotein may facilitate novel coronavirus (SARS-CoV-2) host cell entry [67]. In a study conducted by Kim and coworkers, three GAG-binding motifs were identified. Site 1 in S1 region 453-459 (YRLFRKS), site 2 at S1/S2 proteolytic cleavage site 2681-686 (PRRARS), and site 3 at S2 region 810-816 (SKPSKRS) Figure 3. The binding of the three sites were studied using a surface plasmon resonance direct binding assay, it was found that both monomeric and trimeric SARSCoV- 2 spike more tightly bind to immobilized heparin than the SARS-CoV and MERS-CoV. Later in the same study using unbiased computational ligand docking indicated that HS interacts with the GAG binding motif at the S1/S2 site on each monomer interface in the trimeric SARS-CoV-2 S glycoprotein, and at another site (453- 459 (YRLFRKS)) when the RBD is in an open conformation [17, 67]. The three dimensional structure of the spike protein along with the HS binding domains have been shown in the Figure 4.
Figure 3: SARS-CoV-2, Spike glycoprotein Heparan sulfate: (a) Site 1 in S1 region 453-459 (YRLFRKS); (b) Site 2 at S1/S2 proteolytic cleavage site 2681-686 (PRRARS), and (c) site 3 at S2 region 810-816 (SKPSKRS) binding highlighted in box.
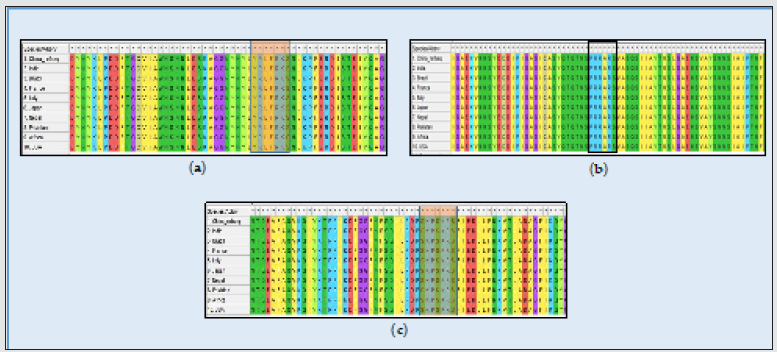
Figure 4: The SARS-CoV-2 spike protein structure showing heparan sulfate binding domain: (a) 453-459 (amino acids YRLFRKS) colored in red; (b) Close up view of the heparan sulfate receptor binding, highlighting domain 453 – 459 (amino acids YRLFRKS) motif at the surface of the domain colored in red. (c) S2 region 810-816 (SKPSKRS) colored in red; (d) Close up view of the heparan sulfate receptor binding highlighting domain 810 - 816 (amino acids SKPSKRS) motif at the surface of the domain colored in red; (e) RGD domain S gene 403 – 405 colored in red; (f) Close up view of the heparan sulfate receptor binding, highlighting domain 403 – 405 (amino acids RGD) motif at the surface of the domain colored in red.
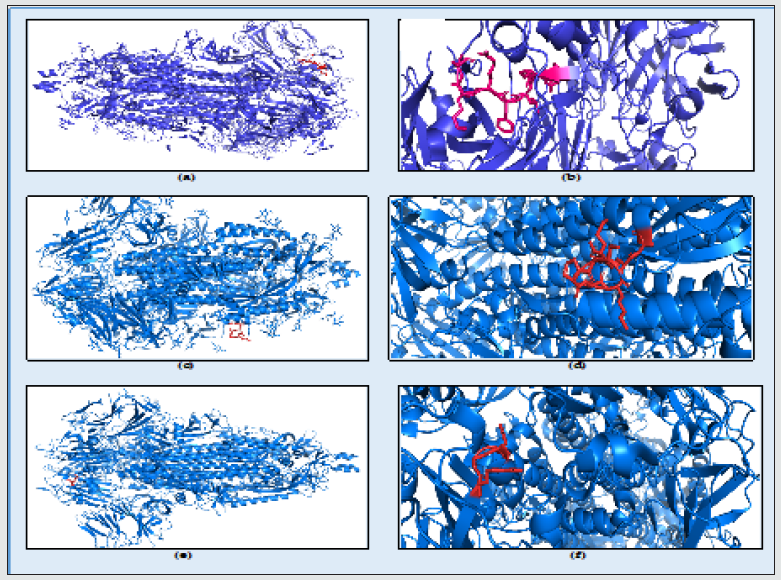
The RBD undergoes conformational change upon interaction with low molecular weight heparins [68]. SARS-CoV-2 S1 RBD binds to heparin and that upon binding, a significant structural change is induced. Moreover, moieties of basic amino acid residues, known to constitute heparin binding domains, are solvent accessible on the SARS-CoV-2 S1 RBD surface and form a continuous patch that is suitable for heparin binding [59]. The dependence of the host on the interaction of hundreds of extracellular proteins with the cell surface GAG molecules for the regulation of homeostasis is exploited by many microbial pathogens as a means of adherence and invasion. The closely related polysaccharide heparin, a widely used anticoagulant drug, which is structurally similar to HS and is a common experimental proxy, can be expected to mimic the properties of HS. Heparin prevents infection by a range of viruses when added exogenously, including S-associated coronavirus strain HSR1 and inhibits cellular invasion by SARS-CoV-2 [59]. It has been previously demonstrated that unfractionated heparin binds to the Spike (S1) protein RBD, induces a conformational change and have reported the structural features of heparin on which this interaction depends. Furthermore, it is demonstrated that enoxaparin, a low molecular weight clinical anticoagulant, also binds the S1 RBD protein and induces conformational change [59]. SARS-CoV-2 uses integrins as cell receptors in one or more host species, binding to them through a conserved RGD (403–405: Arg-Gly-Asp) motif that is present in the RBD of the spike proteins of all SARS-CoV-2 sequences analyzed to date [Figure 5]. The motif was identified by a PROSITE scan that included motifs with a high probability of occurrence (PDOC00016) Table 1 [60].
Table 1: Amino acid moieties responsible for binding of Heparan sulfate receptors with S glycoprotein of SARS-CoV-2.
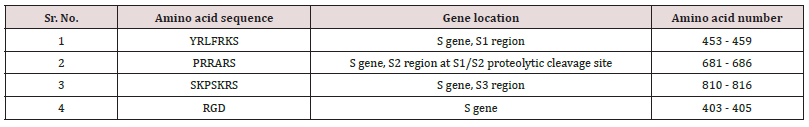
Figure 5: SARS-CoV-2, Spike glycoprotein Heparan sulfate binding RGD (403-405) domain highlighted in box.
Targeting Heparan Sulfate for Therapeutic Applications
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
This review indicated the possibility of Heparan sulfate as playing a significant role in viral entry. Different mechanisms have been suggested over the year to block heparan sulfate to prevent viral binding. These inhibitors can help prevent ocular transmission of the viral particles.
Fibroblast growth factor-2 (FGF-2):
FGF is a strong heparin binding protein. Previous studies have indicated that stromal keratitis caused by ocular herpes virus infection can be diminished by FGF-2. FGF-2 competes with HSV-1 for heparin sulfate [69]. This role of FGF-2 in inhibiting binding of virus and heparin sulfate in ocular surfaces can be extended to treat spread of SARS-CoV 2 through ocular surfaces.
G1 and G2 peptide
G1 and G2 are peptides developed against commercially found HS and specifically modified 3-OS HS respectively. [70]. A phage display peptide library screening method was used to find these peptides to block against HS. This strategy of developing peptides showed promising results as a therapeutic against ocular HSV-1 infection in mouse corneas with no damage to the corneal epithelium. [70] Thus, these peptides might also show promise against ACE2 binding to HS in human corneas for SARS-CoV-2 treatment.
Inhibition by Heparin
Heparin belongs to family of GAG molecules. In humans, heparin is produced exclusively by mast cells and stored there in granules. Mast cells are located in the lungs, gut and along the blood vessels and heparin from these mast cells plays an important role in host defense mechanism. Heparin competes with HS for adhesion of bacteria and viruses and thus inhibits the invasion by pathogen [71]. Heparin can be used to inhibit cellular invasion by SARS-CoV-2 by interaction of the surface protein (spike) S1 RBD with heparin [68]. Studies have also shown inhibition of SARS-CoV-2 entry by bovine Lactoferrin- another HS mimic [72]. Nebulization of heparin for use as therapeutic against SARS-CoV-2 is another promising option [73]. Unfractionated heparin inhibited spike protein binding with an IC50 value of <0.05U/ml. This suggests that heparin, particularly unfractionated forms, could be considered to reduce clinical manifestations of COVID-19 by inhibiting continuing viral infection [16]. Administration of low-molecular-weight heparin is beneficial to patients with severe coronavirus disease 2019 (COVID-19), but the mechanism is unknown. HS may bind to severe acute respiratory syndrome coronavirus 2 spike protein to block viral attachment or entry. HS attenuates inflammation responses through neutralizing the activity of pro-inflammatory proteins, that is, histone and high mobility. Use of specially designed HS oligosaccharides offer a new strategy to manage COVID-19 [17]. Although there are no studies for using heparin at ocular surface for COVID-19, further studies in animal corneas can be used to promote Heparin to block HS for SARS-CoV-2 entry into eyes.
Most severe manifestations of COVID‐19 cases, such as multiple organ failure and death, have been linked to coagulation dysfunction markers, such as platelet reduction and increases in prothrombin time, fibrin degradation products, and, mainly, D‐dimer [74]. A recent paper by Tang et al in this journal reported that heparin treatment reduced mortality of COVID‐19 patients with elevated Ddimer; similar preliminary results have been reported elsewhere [75, 76]. A mounting body of evidence shows that SARS‐CoV‐2 causes a “cytokine storm” that activates the coagulation cascade, leading to thrombosis [77]. Similar to the findings in severe sepsis, generalized deposition of intravascular thrombi compromises the blood supply of several organs, leading to organ failure [78]. A direct anticoagulant effect is likely crucial to the therapeutic effect of heparin, it also has antiarrhythmic properties that shows promise in the treatment of COVID‐19, in which cardiac arrhythmias are the immediate cause of several patient deaths [79]. Heparin had been used in 1734 patients. Heparin was associated with lower mortality when the model was adjusted for age and gender, with OR (95% CI) 0.55 (0.37–0.82) p=0.003. This association remained significant when saturation of oxygen<90%, and temperature>37 °C were added to de model with OR 0.54 (0.36–0.82) p=0.003, and also when all the other drugs were included as covariates OR 0.42 (0.26–0.66) p<0.001. The association between heparin and lower mortality observed in the study by Ayerbe et al can be acknowledged by clinicians in hospitals and in the community [80]. In an another clinical study conducted by Shi et al the D-dimer, C reactive protein CRP and PBMC (peripheral blood mono-nuclear cells percentage, IL-6 and other parameters was analyzed in patients with low molecular weight heparin (LMWH) treatment and control group. Under conventional antiviral treatment regimens, LMWH can improve hypercoagulability, inhibit IL-6 release, and counteract IL-6 biological activity in patients. LMWH has potential antiviral effects and can help delay or block inflammatory cytokine storms. It can also increase the lymphocytes (LYM%) of patients and has the potential for treatment of COVID-19 [81]. As per our literature search, no concrete data is available on the ocular therapeutic usage of these molecules/drugs. Only limited studies have been done in relation to ocular manifestation of COVID-19.
GAG mimetics
Synthetic GAG mimetics are polyanionic molecules that inhibit HS-protein interactions. Heparin polysaccharides are digested to generate GAG derivatives. Heparin are good inhibitors too and have been able to prevent binding of viruses like HSV [82] and HPV [64]. But it’s anticoagulant characteristics induce thrombocytopenia. To overcome these problems, GAG mimetics were developed. Examples of GAG derivatives that have been developed are non-sulfated K5 polysaccharide from E. coli and 3-O-sulfated octasaccharide which is like 3-O-sulfate domain of HS. These have been successful in blocking viral infections. Blocking H5N1 influenza virus attachment by modified heparin mimetics showed promising results. Desuflation of this modified heparin reduced the anticoagulant effect without hampering the inhibition. Other potent antiviral GAG mimetics with effective viral inhibitions are rhamnan sulfate, PG545, and PI-88. Sulfated polysaccharides similar to HS isolated from seaweed have been effective in inhibiting HSV virus and Dengue. GAG derivatives are promising as antiviral in cell cultures. In vivo testing in corneas is required to use these as antiviral for humans in future. PI-88 and PG545 were in clinical trials for treating tumors. But due to its toxic effects, the trial was discontinued [83]. Better and safer GAG derivatives directed towards ocular surfaces might have potential as a SARS-CoV 2 antiviral for ocular manifestation.
Discussion
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
There is an increasing number of scientific reports regarding conjunctivitis as an ocular manifestation of COVID-19. Given the history of coronaviruses found in tears or conjunctival swabs, scientists believe that SAR-CoV-2 should also display a similar phenomenon. Many institutes are trying to collect more data to corroborate this theory. Although growing number of publications are reporting ocular manifestation case studies, the data with coronavirus patients with conjunctivitis is still rare. The presence of various viral receptors on the ocular surface such as ACE2 and HS do indicate the probability of ocular transmission of Covid-19, but the actual human data is limited in number and scope to support this theory. The relatively weaker binding of ACE2 on ocular surface indicates that the virus may only transiently stay there during an early infection and then move to the nasolacrimal duct via the tear fluid to reach the lungs where it binds strongly to the cells expressing ACE2 and HS to facilitate the infection and subsequently, the respiratory disorders. The data till now indicates that understanding these ocular manifestations are secondary in priority. However, with this epidemic growing, each piece of information is important to find possible treatment and prevent its transmission.
Acknowledgement
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
This work was supported by NIH grants (R01 EY024710 and P30 EY001792) to D.S.
Conflict of Interest
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
There is no economic interest or conflict of interest.
References
- Abstract
- Introduction
- SARS CoV-2 Host Cell Entry
- Ocular Viral Transmission
- Conjunctivitis and Its Correlation with Coronavirus
- Receptors Involved in Ocular Manifestations
- Role of Heparan sulfate in Ophthalmic manifestation of COVID-19
- Targeting Heparan Sulfate for Therapeutic Applications
- Discussion
- Acknowledgement
- Conflict of Interest
- References
- Fehr AR, HJ PS, Bickerton E, Britton P (2015) An Overview of Their Replication and Pathogenesis; Section 2 Genomic Organization. Methods in Molecular Biology. Springer 1282: 1-23.
- Galbadage T, Peterson BM, Gunasekera RS (2020) Does COVID-19 spread through droplets alone? Frontiers in public health 24(8): 163.
- Dockery DM, Rowe SG, Murphy MA, Krzystolik MG (2020) The ocular manifestations and transmission of COVID-19: recommendations for prevention. The Journal of emergency medicine 59(1): 137-140.
- Panoutsopoulos AA (2020) Conjunctivitis as a sentinel of SARS-CoV-2 infection: a need of revision for mild symptoms. SN Comprehensive Clinical Medicine 19: 1-6.
- Loon SC, Teoh SC, Oon LL, Se Thoe SY, Ling AE, et.al. (2004) The severe acute respiratory syndrome coronavirus in tears. British journal of ophthalmology 1;89(3): 861-863.
- Lu R, Zhao X, Li J, Niu P, Yang B (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The lancet 395(10224): 565-574.
- Raboud J, Shigayeva A, McGeer A, Bontovics E, Chapman M, et.al. (2010) Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PloS one 5(5): e10717.
- Hu K, Patel J, Swiston C, Patel BC (2021) Ophthalmic manifestations of coronavirus (COVID-19). StatPearls.
- Bi Q, Wu Y, Mei S, Ye C, Zou X, et.al. (2020) Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. The Lancet Infectious Diseases 20(8): 911-919.
- Shang J, Wan Y, Luo C, Ye G, Geng Q, et.al. (2020) Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences 117(21): 11727-11734.
- Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, et.al. (2020) SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 183(4): 1043-1057.
- Wan Y, Graham R, Baric R, Li F (2020) An analysis based on decade-long structural studies of SARS 3, JVI Accepted Manuscript Posted Online J. Virol.
- Bestle D, Heindl MR, Limburg H, Pilgram O, Moulton H (2020) TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life science alliance 1: 3(9).
- Hoffmann M, Kleine Weber H, Schroeder S, Krüger N, Herrler T (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor cell. 181(2): 271-280.
- Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J (2014) Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. Journal of virology 88(22): 13221-13330.
- Partridge LJ, Urwin L, Nicklin MJ, James DC, Green LR, et.al. (2020) ACE2-independent interaction of SARS-CoV-2 spike protein to human epithelial cells can be inhibited by unfractionated heparin. bioRxiv.
- Liu L, Chopra P, Li X, Wolfert MA, Tompkins SM (2020) SARS-CoV-2 spike protein binds heparan sulfate in a length-and sequence-dependent manner. BioRxiv.
- Wang J, Du G (2020) COVID-19 may transmit through aerosol. Irish Journal of Medical Science 24: 1-2.
- Belser JA, Rota PA, Tumpey TM (2013) Ocular tropism of respiratory viruses. Microbiology and Molecular Biology Reviews 77(1): 144-156.
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GV, et.al. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland 203(2): 631-637.
- Sun C, Wang Y, Liu G, Liu Z (2020) Role of the eye in transmitting human coronavirus: what we know and what we do not know. Front Public Health 24;8: 155.
- Sun Y, Liu L, Pan X, Jing M (2006) Mechanism of the action between the SARS-CoV S240 protein and the ACE2 receptor in eyes. Int J Ophthalmol 6(4): 783-786.
- Petronio GP, Di Marco R, Costagliola C (2020) Do Ocular fluids represent a transmission route of SARS-CoV-2 infection? Frontiers in Medicine5;7: 620412.
- Tong TR, Lam BH, Ng TK, Lai ST, Tong MK, et.al. (2003) Conjunctiva-upper respiratory tract irrigation for early diagnosis of severe acute respiratory syndrome. Journal of clinical microbiology 41(11): 5352.
- Seah I, Agrawal R (2020) Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocular immunology and inflammation 2;28(3): 391-395.
- Seah IY, Anderson DE, Kang AE, Wang L, Rao P, et.al. (2020) Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology 127(7): 977-979.
- Azari AA, Barney NP (2013) Conjunctivitis: a systematic review of diagnosis and treatment. Jama 310(16): 1721-1729.
- Xia J, Tong J, Liu M, Shen Y, Guo D (2020) Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS‐CoV‐2 infection. Journal of medical virology 92(6): 589-594.
- Vabret A, Mourez T, Dina J, Van Der Hoek L, Gouarin S, et.al. (2005) Human coronavirus NL63, France. Emerging infectious diseases 11(8): 1225.
- Chan WM, Yuen KS, Fan DS, Lam DS, Chan PK, et.al. (2004) Tears and conjunctival scrapings for coronavirus in patients with SARS. British Journal of Ophthalmology 88(7): 968-969.
- Deng W, Bao L, Gao H, Xiang Z, Qu Y, et.al. (2020) Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nature communications 11(1): 4400.
- Willcox MD, Walsh K, Nichols JJ, Morgan PB, Jones LW (2020) The ocular surface, coronaviruses and COVID‐ Clinical and Experimental Optometry 103(4): 418-24.
- Zhou Y, Zeng Y, Tong Y, Chen C (2020) Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. MedRxiv.
- Imai M, Iwatsuki Horimoto K, Hatta M, Loeber S, Halfmann PJ, et.al. (2020) Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proceedings of the National Academy of Sciences 117(28): 16587-16595.
- Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, et.al. (2020) Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clinical Infectious Diseases 71(9): 2428-2446.
- Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, et.al. (2020) Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Canadian journal of ophthalmology 55(4): e125-e129.
- Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, et.al. (2020) SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Annals of Internal Medicine 173(3): 242-243.
- Dai X (2020) Peking University Hospital Wang Guangfa disclosed treatment status on Weibo and suspected infection without wearing goggles. Beijing News 22: 24.
- Zhou Y, Zeng Y, Tong Y, Chen C (2020) Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. MedRxiv.
- Zhang X, Chen X, Chen L, Deng C, Zou X, et.al. (2020) The evidence of SARS-CoV-2 infection on ocular surface.18(3): 360-362.
- Xia J, Tong J, Liu M, Shen Y, Guo D (2020) Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS‐CoV‐2 infection. Journal of medical virology 92(6): 589-594.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. New England journal of medicine 58(4): 711-712.
- Wu P, Duan F, Luo C, Liu Q, Qu X, et.al. (2020) Characteristics of Ocular Findings of Patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol 138: 575-578.
- Chen L, Deng C, Chen X, Zhang X, Chen B, et.al. (2020) Ocular manifestations and clinical characteristics of 535 cases of COVID‐19 in Wuhan, China: a cross‐sectional study. Acta ophthalmologica 98(8): e951-9.
- Al-Sharif E, Strianese D, AlMadhi NH, D’Aponte A, dell’Omo R, et.al. (2021) Ocular tropism of coronavirus (CoVs): a comparison of the interaction between the animal-to-human transmitted coronaviruses (SARS-CoV-1, SARS-CoV-2, MERS-CoV, CoV-229E, NL63, OC43, HKU1) and the eye. International ophthalmology 41(1): 349-362.
- Sarma P, Kaur H, Kaur H, Bhattacharyya J, Prajapat M, et al. (2020) Ocular Manifestations and Tear or Conjunctival Swab PCR Positivity for 2019-nCoV in Patients with COVID-19: A Systematic Review and Meta-Analysis.
- Loffredo L, Pacella F, Pacella E, Tiscione G, Oliva A, et al. (2020) Conjunctivitis and COVID-19: a meta-analysis. J Med Virol 92(9): 1413-1414.
- Siedlecki J, Brantl V, Schworm B, Mayer WJ, Gerhardt M, et al. (2020) COVID-19: ophthalmological aspects of the SARS-CoV 2 global pandemic. Klin Monbl Augenheilkd 237(5): 675-680.
- Emparan JPO, Sardi Correa C, López Ulloa JA, Viteri Soria J, Penniecook JA, et al. (2020) COVID-19 and the eye: how much do we really know? A best evidence review. Arq Bras Oftalmol 83(3): 250-261.
- Torres Costa S, Lima Fontes M, Falcão Reis F, Falcão M. (2020) SARS-COV-2 in ophthalmology: current evidence and standards for clinical practice. Acta Méd Port 33(9): 593-600.
- Bonn D (2004) SARS virus in tears? The Lancet Infect Dis 4(8): 480.
- Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C (2020) The protein expression profile of ACE2 in human tissues. Mol Syst Biol 16(7): e9610.
- Ma D, Chen CB, Jhanji V, Xu C, Yuan XL, et al. (2020) Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye(Lond) 34(7): 1212-1219.
- Jeffers SA, Tusell SM, Gillim Ross L, Hemmila EM, Achenbach JE, et al. (2004) CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA 101(44): 15748-15753.
- Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, et al. (1992) Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 357(6377): 420-422.
- Milewska A, Nowak P, Owczarek K, Szczepanski A, Zarebski M, et al. (2018) Entry of human coronavirus NL63 into the cell. J Virol 92(3): e01933-17.
- Cagno V, Tseligka ED, Jones ST, Tapparel C (2019) Heparan sulfate proteoglycans and viral attachment: true receptors or adaptation bias? Viruses 11(7): 596.
- WuYang ZH, JiangJiao LI, Liang G (2011) How does cellular heparan sulfate function in viral pathogenicity? Biomedical and Environmental Sciences 24(1): 81-87.
- Gallagher JT (2001) Heparan sulfate: growth control with a restricted sequence menu. J Clin Invest 108(3): 357-361.
- Iozzo RV (1998) Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 67(1): 609-652.
- Agelidis A, Shukla D (2020) Heparanase, heparan sulfate and viral infection. Adv Exp Med Biol 1221: 759-770.
- Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, et al. (1997) Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med 3(8): 866-871.
- Shukla D, Spear PG (2001) Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest 108(4): 503-510.
- Giroglou T, Florin L, Schäfer F, Streeck RE, Sapp M (2001) Human papillomavirus infection requires cell surface heparan sulfate. Journal of virology 75(3): 1565-1570.
- Buzza MS, Zamurs L, Sun J, Bird CH, Smith AI, et al. (2005) Extracellular matrix remodeling by human granzyme B via cleavage of vitronectin, fibronectin, and laminin. J Biol Chem 280(25): 23549- 23558.
- Hallak LK, Spillmann D, Collins PL, Peeples ME (2000) Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J virol 74(22): 10508-10513.
- Kim D, Lee JY, Yang JS, Kim JW, Kim VN, et al. (2020) The architecture of SARS-CoV-2 transcriptome. Cell 181(4):914-921.
- Mycroft West CJ, Su D, Elli S, Li Y, Guimond SE, et al. (2020) The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. BioRxiv.
- Sigrist CJ, Bridge A, Le Mercier P (2020) A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res177: 104759.
- Kim B, Lee S, Kaistha SD, Rouse BT (2006) Application of FGF-2 to modulate herpetic stromal keratitis. Curr Eye Res. 31(12): 1021-1028.
- Tiwari V, Liu J, Valyi Nagy T, Shukla D (2011) Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J Biol Chem 286(28): 25406-25415.
- Ernst S, Langer R, Cooney CL, Sasisekharan R (1995) Enzymatic degradation of glycosaminogIycans. Crit Rev Biochem Mol Biol 30(5): 387-444.
- Pojasek K, Shriver Z, Hu Y, Sasisekharan R (2000) Histidine 295 and histidine 510 are crucial for the enzymatic degradation of heparan sulfate by heparinase III. Biochemistry 39(14) :4012-4019.
- Basu Ray I, Soos MP (2020) Cardiac manifestations of coronavirus (COVID‐19). StatPearls.
- Tang N, Bai H, Chen X, Gong J, Li D, Sun Z (2020) Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 18(5): 1094- 1099.
- Negri EM, Piloto BM, Morinaga LK (2020) Heparin therapy improving hypoxia in COVID‐19 patients ‐ a case series. São Paulo. Front Physiol 11: 573044.
- Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C (2020) Cardiac and arrhythmic complications in patients with COVID‐ J Cardiovasc Electrophysiol 31(5): 1003-1008.
- Burzynski LC, Humphry M, Pyrillou K (2019) The coagulation and immune systems are directly linked through the activation of interleukin‐1α by Thrombin. Immunity 50(4): 1033-1042.e6.
- Menezes Rodrigues FS, Padrão Tavares JG, Pires de Oliveira M, Guzella de Carvalho R, Ruggero Errante P, et al. (2020) Anticoagulant and antiarrhythmic effects of heparin in the treatment of COVID‐19 patients. J Thromb Haemost 18(8): 2073-2075.
- Ayerbe L, Risco C, Ayis S (2020) The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis 50(2): 298-301.
- Shi C, Wang C, Wang H, Yang C, Cai F, et al. (2020) Clinical observations of low molecular weight heparin in relieving inflammation in COVID-19 patients: A retrospective cohort study. medrxiv.
- Koganti R, Suryawanshi R, Shukla D (2020) Heparanase, cell signaling, and viral infections. Cell Mol Life Sci 77(24): 5059-5077.
- Laquerre S, Argnani R, Anderson DB, Zucchini S, Manservigi R, et al. (1998) Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol 72(7): 6119-6130.




