
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Case Report(ISSN: 2637-6628) 
Uncontrolled Seizures and De Novo Onset Mesial Temporal Sclerosis on MRI Volume 6 - Issue 4
Joel M Oster1* and G Rees Cosgrove2
- 1Department of Neurology at Tufts Medical Center/ Tufts University, USA
- 2Department of Neurosurgery, Harvard Medical School, Brigham and Women’s Hospital, USA
Received: October 10, 2022; Published: October 27, 2022
Corresponding author: Department of Neurology at Tufts Medical Center/ Tufts University, 800 Washington St Box 314, Boston MA 02111, USA
DOI: 10.32474/OJNBD.2022.06.000241
Abstract
Background
Mesial temporal sclerosis and hippocampal atrophy are frequently associated with febrile convulsions in childhood or severe, frequent and recurrent seizures of the temporal lobe in the pediatric population. The exact mechanisms and factors, number and types of seizures involved, and the chronicity of this process are among the unknown variables. The observation of such cases developing de novo in the human adult clinical population has been rarely documented.
Keywords: Epilepsy; Intractable Epilepsy; Status Epilepticus; Medication Non-Compliance; Mesial Temporal Sclerosis; Neuroimaging; Hippocampal Atrophy
Objective
This paper identifies a case of adult-onset mesial temporal sclerosis and describes the clinical features and MRI findings of this unusual condition.
Method of Study
Retrospective case report.
Setting
A suburban Neurology/Epilepsy Outpatient and Inpatient clinic and hospital.
Findings
This unique case report illustrates the onset of hippocampal atrophy and changes consistent with mesial temporal sclerosis (MTS) on 1.5 T MRI in a 48-year-old adult with adult onset epilepsy. The development of changes consistent with MTS on 1.5 T MRI occurred de novo within a 20-month period of seizure onset. This patient has frequent partial seizures, complex partial seizures, secondarily generalized convulsions, and a clinical history consistent with multiple episodes of probable status epilepticus after suffering an ipsilateral intracranial hemorrhage.
Presentation of Case
A 48 year old right handed male without history or family history of seizures with normal neurodevelopment and essential hypertension presented to another hospital in May 2007 with acute onset of dizziness, headache, slow speech, and rightsided weakness. He generally did not take his antihypertensive medications, although his hypertension was well controlled while on medications. He previously had elective gastric by-pass surgery that was performed due to severe obesity and he lost approximately 100 lbs subsequently. Initial CT scan demonstrated a left parietaloccipital intracerebral hemorrhage. The patient was started on leviteracetam. After a 3-day hospitalization he was discharged.
The intracerebral hemorrhage was deemed possibly related to underlying uncontrolled hypertension and seemed atypical for a post-traumatic hemorrhage. After 1 week, he took medications erratically, and began having increased language difficulty and right-sided weakness. The family contacted emergency medical services and he was transported to our facility. Upon arrival, he became unresponsive, and stiffened the extremities with clinical seizure activity.
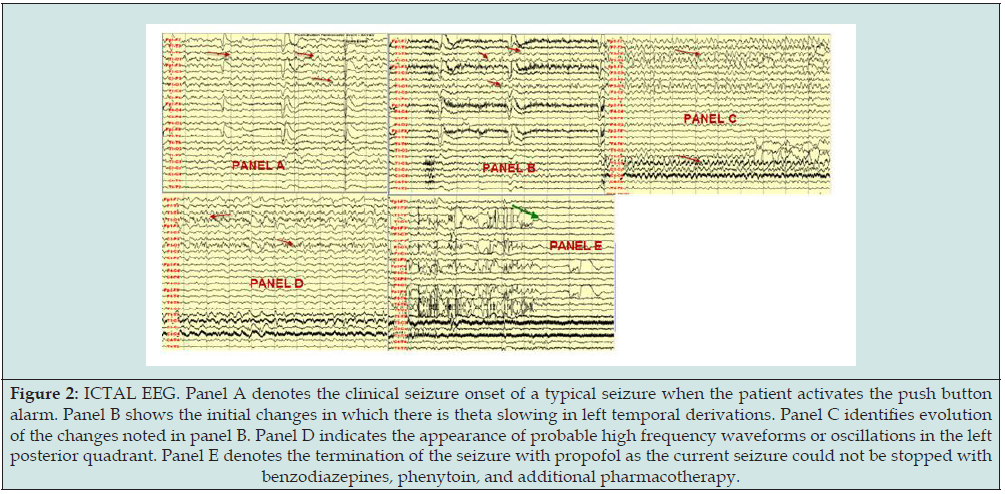
He was intubated, and begun on propofol. MRI demonstrated the noted subacute intracerebral hemorrhage in the left parietaloccipital region with mild mass effect (Figure 1) but CT-angiogram demonstrated no underlying vascular malformation. (Not Shown). Subsequently, to exclude CNS vasculitis or other vasculopathy, cerebral angiography was performed and was normal. (Figures not shown). Ultimately the patient was extubated and the patient reached baseline. He was discharged on oral leviteracetam to home but exhibited frequent seizures approximately every 6 weeks afterwards due to medication non-compliance. A typical seizure stated with a vague feeling of “feeling drunk” followed by arrest of activity, staring, and language difficulty lasting minutes. He would progress to be somnolent or he might exhibit right eye deviation, and tonic-clonic activity of his right extremities which then could progress to involve all four extremities followed by somnolence. Stress and fatigue seemed to increase these events. It took many minutes for him to lose consciousness completely, and he might have language difficulty lasting up to approximately 24 hours after events. The patient describes a history of having several longer episodes of cognitive dysfunction prior to probable secondary generalization and likely complex partial status epilepticus. For full management he was electively admitted to the hospital for inpatient video EEG monitoring (Figure 2).
Figure 1: Top Row : 1.5 T MRI Imaging denoting the presence of a left posterior quadrant hemorrhage. Left panel shows an Axial T1 weighted image with gadolinium enhancement, middle panel and right panels show an axial gradient echo image. Left 2 panels are from the initial MRI in 2007, and the right panel is from an MRI series in 2011. Middle and Bottom Rows: MRI Imaging showing the development of progressive predominantly left hippocampal atrophy. Middle panels are 1.5 T Contrast Enhanced SPGR Coronal thin sections through the anterior portions of the hippocampi. Bottom panels are 1.5 T T2 Coronal thin sections. From left to right, imaging obtained in 2007 followed by that in 2009 and 2011 are shown at the approximately similar section slice. Red Arrows denote the progressive change in appearance particularly of the left hippocampus over time that becomes atrophied and contains more CSF density surrounding it indicative of progressive volume loss. Although quantitative volumetric analysis could not be done, these visually noted features are consistent with the development of Mesial Temporal Sclerosis (MTS) over approximately a 20 month period.
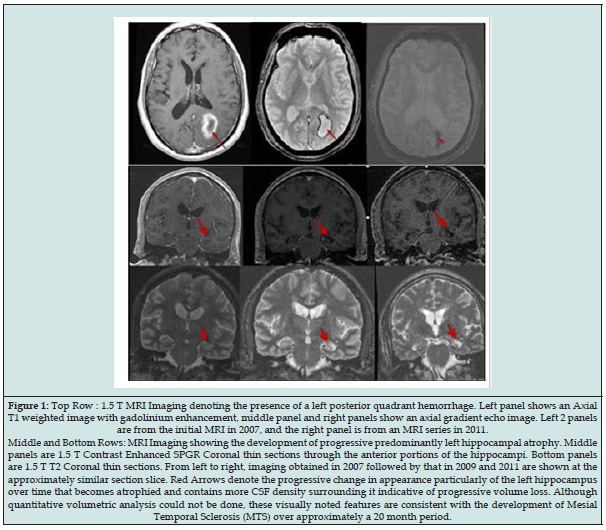
Figure 2: ICTAL EEG. Panel A denotes the clinical seizure onset of a typical seizure when the patient activates the push button alarm. Panel B shows the initial changes in which there is theta slowing in left temporal derivations. Panel C identifies evolution of the changes noted in panel B. Panel D indicates the appearance of probable high frequency waveforms or oscillations in the left posterior quadrant. Panel E denotes the termination of the seizure with propofol as the current seizure could not be stopped with benzodiazepines, phenytoin, and additional pharmacotherapy.
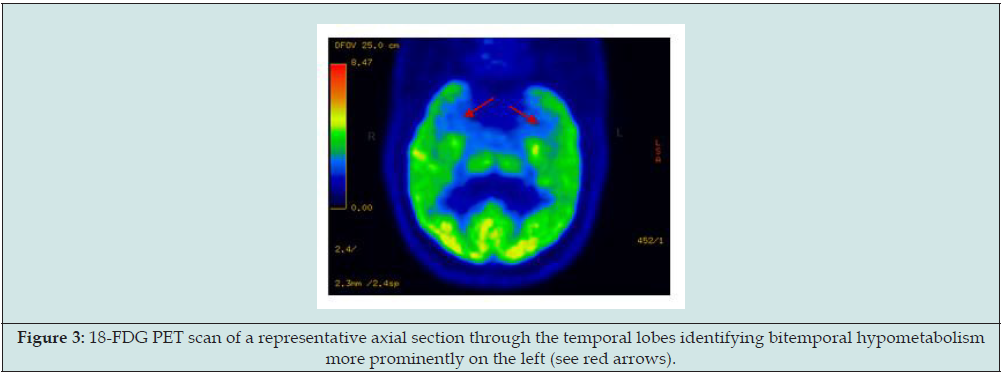
To date he has been administered the following medications for seizures: phenytoin, lamotrigine, zonisamide, leveteracetam, pregabalin, and lacosamide in various combinations. The patient declined other options including surgery. His reason for declining other therapies is that he believes medications actually work when he takes them and therefore the disorder seems not to be highly pharmacoresistant although he is noncompliant. Serial 1.5 T Brain MRI neuroimaging done starting from in 2007 through 2012 shows that in approximately 20 months after the onset of the seizures, MRI findings develop consistent with left mesial temporal sclerosis and hippocampal atrophy (Figure 1). A Brain PET scan indicated that there was evidence of bilateral but predominantly left temporal lobe hypometabolism or dysfunction (Figure 3).
Figure 3: 18-FDG PET scan of a representative axial section through the temporal lobes identifying bitemporal hypometabolism more prominently on the left (see red arrows).
Discussion
Recurrent seizures are often caused by lesions particularly in the temporal lobe and recurrent seizures may induce morphological changes in the brain if they recur to a frequent or severe enough degree [1-4]. The exact mechanisms and factors, number and types of seizures involved, and the chronicity of this process are among the unknown variables. It has been noted that MTS is the single pathology found in approximately two-thirds or more of patients with temporal lobe epilepsy and MTS is characterized by selective neuronal loss and reactive gliosis in the hippocampus and other mesial limbic structures [4-9]. While MTS is noted to be a common pathologic finding in patients with temporal lobe epilepsy, particularly cases that had onset of seizures as young children, MTS however is not commonly identified until adolescence or young adult life when seizures have become intractable after many years [4-9]. While the literature notes that status epilepticus is associated with MTS, many of the reports are of cases with encephalitis and severe complications such as hypoxia, hypoglycemia, or with major other systemic illnesses [7-9]. In the absence of a systemic disturbance or other underlying cause, it seems that the abnormalities detected on the MRI series in this case are a direct result of repeated seizures. While a rare de novo adult onset hippocampal sclerosis syndrome is possible, generally these cases are pharmacoresistant which is unlike the current case [10].
Another possibility is that the seizures might be related to the intracranial hemorrhage, vasculopathy, or “dual” pathology since temporal onset seizures may result from remote lesions and occipital onset seizures can spread to the temporal lobe [11-13]. In our patient with the noted clinical presentation, EEG monitoring proved that he did not have extratemporal onsets. Furthermore, the incidence of seizures after stroke or hemorrhage in the deep white matter without major cortical involvement is generally low [13-15]. Although other diagnoses could be possible as part of a more systemic illness such as a vasculopathy or vasculitis, clinically the patient enjoys otherwise good health and does not have any evidence of a more widespread evolving or subacute systemic vasculitis or central nervous system vasculitis over a multiyear follow up period and had a negative cerebral angiogram.
In summary, this unique case report illustrates the onset of changes on 1.5 T Brain MRI consistent with new onset MTS and hippocampal atrophy in an adult patient with frequent seizures and medication noncompliance, with clinical factors noted above. The presence of an ipsilateral intracerebral hemorrhage may be a contributing factor due to an unknown mechanism, but the overall mechanisms of epileptogenesis and ultrastructural change noted on the MRI remain uncertain. While historically MTS has been noted to be a pediatric disease, this case report documents rare de novo adult-onset hippocampal atrophy consistent with MTS on visual inspection of an MRI series over an approximate 20-month follow up period. This adult onset de novo finding over 20 months is What this report wishes to report as a significant and unique finding.
References
- Cole AJ (2004) Status epilepticus and peri-ictal imaging. Epilepsia 45(Suppl 4): 72-77.
- Doherty CP, Cole AJ, Grant PE, Fischman A, Dooling E, et al. (2004) Multimodal longitudinal imaging of focal status epilepticus. Can J Neurol Sci 31(2): 276-281
- Oster J, Doherty C, Grant PE, Simon M, Cole AJ (2003) Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia 44(6): 852-854.
- Liu Z, Mikati M, Holmes GL (1995) Mesial temporal sclerosis: Pathogenesis and significance. Pediatr Neurol 12(1): 5-16.
- French JA, Williamson PD, Thadani VM, TM Darcey, RH Mattson, et al. (1993) Characteristics of medial temporal lobe epilepsy : I. Results of history and physical examination. Ann Neurol 34(6): 774-780.
- Williamson PD, French JA, Thadani VM, JH Kim, RA Novelly, et al. (1993) Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol 34(6): 781-787.
- Parmar H, Lim SH, Tan NC, Lim (2006) Acute Symptomatic Seizures and Hippocampus Damage: DWI and MRS Findings. Neurology 66(11): 1732-1735.
- Lewis DV, Barboriak DP, MacFall JR, Provenzale JM, Mitchell TV, et al. (2002) Do prolonged febrile seizures produce medial temporal sclerosis? Hypotheses, MRI evidence and unanswered questions. Prog Brain Res 135: 263-278.
- Provenzale JM, Barboriak DP, VanLandingham KE, MacFall J, Delong D, et al. (2008) Hippocampal MRI signal hyperintensity after febrilestatus epilepticus. Am J Roentgenol 190(4): 976-983.
- Labate A, Ventura P, Gambardella A, E Le Piane, E Colosimo, et al. (2006) MRI evidence of mesial temporal sclerosis in sporadic “benign” temporal lobe epilepsy. Neurology 66(4): 562-565.
- Li LM, Candes F, Anderman F, Watson C, Fish DR, et al. (1999) Surgical outcome in patients with epilepsy and dual pathology. Brain 122(Pt 5): 799-805.
- Salanova V, Andermann F, Olivier A, T Rasmussen, LF Quesney (1992) Occipital lobe epilepsy: Electroclinical manifestations, electrocorticography, cortical stimulation, and outcome in 42 patients treated between 1930 and 1991. Brain 115: 1655-1680.
- Morioka T, Nishio S, Hisada K, M Muraishi, H Ishibashi, et al. (1998) Two cases of mesial temporal lobe epilepsy associated with old intracerebral hemorrhage in the lateral temporal lobe without dual pathology. No ShinkeiGeka 26(5): 449-456.
- Beghi E, D Alessandro R, Beretta S, D Consoli, V Crespi, et al. (2011) Incidence and predictors of acute symptomatic seizures after stroke. Neurology 77(20): 1785-1793.
- De Herdt V, Dumont F, Henon H, P Derambure, K Vonck, et al. (2011) Early seizures in intracerebral hemorrhage: Incidence, associated factors, and outcome. Neurology 77(20): 1794-1800.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




