
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Mini Review(ISSN: 2637-6628) 
Targeted therapy for GBM using CAR T-Cells and CMV Volume 5 - Issue 4
Felix Corr1,2*, Philipp Einheuser1, Beat Alessandri1, Florian Ringel1 and Harald Krenzlin1
- 1Department of Neurosurgery, Johannes Gutenberg-University, Germany
- 2EDU Medical College, Villa Bighi, Chaplain’s House Kalkara, Malta
Received:May 17, 2021 Published:May 21, 2021
Corresponding author:Felix Corr, Department of Neurosurgery, Johannes Gutenberg-University, Langenbeckstrase 1, 55131 Mainz, Germany
DOI: 10.32474/OJNBD.2021.05.000221
Abstract
Glioblastoma is the most common malignant brain tumor associated with poor prognosis and mortality. Despite optimal primary therapy, median survival remains poor. New approaches to tumor eradication are lacking and urgently needed to improve patient survival and quality of life. Immunotherapies promise new treatment strategies in addition to standard treatment. Checkpoint inhibition and programmed death ligand 1 blockage so far have failed to mount a clinical impact in glioblastoma. Modified T-cells are seen as a novel promising therapy as they have not been extensively tested in glioblastoma. Today, such modified T-cells are established in the treatment hematological malignancies. Approaches include adoptive transfer of T-cells genetically engineered with a receptor that targets tumor-associated antigens (TAAs).
Chimeric antigen receptor generated by transferring domains derived from antibodies and T-cell receptors that interact with specific TAAs can provide specific recognition of TAAs. To overcome obstacles in terms of specificity of the TCRs, single chain T-cell receptors might be a promising approach. The role of cytomegalovirus (CMV) for immunotherapy is debated in the literature. Establishment of CMV as a potential antigen for targeted immunotherapy in glioblastoma could be a promising approach in combination with CAR T-cell therapy. In this context, single chain fragment variable may play an important role in the recognition of target antigens. We sought to examine the current state of the literature with respect to CAR T-cell therapy for glioblastoma associated with a CMV positive serotype. Clinically, such an association could lead to the production of new experimental therapies.
Keywords: Glioblastoma; GBM; CAR T-cell; Chimeric Antigen Receptor; T-lymphocytes; Cytomegalovirus; CMV; Immunotherapy; Targeted Therapy; Central Nervous System Neoplasms
Introduction
Among the group of brain-derived tumors, glioblastoma stands out as the most malignant tumor among astrocytoma in terms of both progression free survival, overall survival, mortality and is associated with high economic costs [1-2]. Complexity of neurooncological treatment is evident from the multimodal initial therapy, although the course of therapy is individualized, especially with regard to radio- and chemotherapy, due to histopathological characteristics of tumor biology such as MGMT promoter status [3,4]. Moreover, intratumorally heterogeneity [5], blood brain barrier [6] and immune evasion pose particular challenges for therapeutic approaches [7]. High morbidity and mortality of the tumor therefore requires further experimental approaches, which can be considered as additional therapy options besides the actual standard treatment. The role of immunotherapy is a current point of discussion due to the observed immunosuppressive role of glioblastoma [8]. Immunotherapies including checkpoint inhibition and programmed death ligand 1 (PD-L1) blockage so far have failed to mount a clinical impact in glioblastoma [9].
Experimental studies showed the release of anti-inflammatory cytokines, reducing levels of antigen-presenting molecules, and engaging immune checkpoint–activating regulatory receptors on T-cells via PD-L1. Glioblastoma derived extracellular vesicle (EVs) carrying PD-L1 are able to prevent T-cell activation mediating antitumor immunity suppression [7]. While T-cells are present in glioblastoma, programmed cell death protein 1 (PD-1) blockade did not lead to sufficient T-cell activation and failed to demonstrate anti-tumoral efficacy in phase III clinical trials [9]. T-cell activation and anti-tumor response represents the goal of targeted immunotherapy. Short dendritic cells (DCs) present captured tumor associated antigens to prime and subsequently activate T-cells. Tumor infiltration of cytolytic T-cells with TCR recognition of tumor antigens leads to tumor destruction with a subsequent release of more TAAs for DCs to present [10]. The adoptive transfer of genetically engineered T-cells with a receptor targeting a specific TAA bypassing the education of antigen specific T-cells by DCs establishes another valuable concept for immunotherapeutic approaches. These T-cells use a chimeric antigen receptor which is generated by transferring domains derived from antibodies and T-cell receptors known to interacting with specific TAA [11-13].
Currently, modified T-cells are established in the treatment of acute lymphoblastic leukemia [14], chronic lymphocytic leukemia [15], B-cell lymphoma [16] and multiple myeloma [17]. However, problems in the engineering of CAR T-cells have to be considered. First, identification of a suitable tumor antigen is not distinct. Mispairing of exogenous TCR chains with endogenous TCR chains could lead to a graft-versus-host reaction [18]. To avoid this, single chain T-cell receptor could lead to the elimination of heterodimers, increasing the specificity of targeting specific antibodies [19, 20]. The interface of the immunosuppressive role of glioblastoma and possible immunotherapy with CAR T-cells is potentially filled by the presence of the cytomegalovirus. Human herpesvirus 5 (CMV, cytomegalovirus) remains after infection in the human body throughout life, so that periodic reactivations may occur due to stress and immunocompromise [21].
Prevalence of CMV infection in the general population is estimated between 45% and 100% [22]. Of interest, proteins and nucleic acids of human cytomegalovirus have been detected in several tumor types, including colon cancer [23], prostate cancer [24] and glioblastoma [25, 26]. While proteins were found in the tumor area, they are undetectable outside the tumor in the brain [26]. With respect to an immunosuppressed microenvironment, reactivation of CMV from the latent phase seems likely to occur. Thus, CMV infection is central to tumor biology processes in terms of cell proliferation, invasion and angiogenesis [27]. Various clinical data already propagate the clinical significance for the possible approach of immunotherapy in relation to cytomegalovirus. Thus, CMV could be established as a possible antigen for targeted immune-therapy in glioblastoma using CAR T-cells.
Results
Targeted Immunotherapy using Chimeric Antigen Receptor T-Cells
CAR T-cells reveal a promising approach in immunotherapy. To date, there are only a few clinically approved applications of CAR T-cells. In the treatment of B-cell precursor acute lymphoblastic leukemia, tisagenlecleucal, a CD19-targeted CAR T cell therapy, has been approved [28]. Clinical evidence is very limited in glioblastoma, with mainly experimental in vitro and in vivo studies. The main hurdles in T-cell therapy in glioblastoma are considered to be tumor heterogeneity, blood-brain barrier, immunosuppressive microenvironment and antigen escape [29]. As a new target, fibroblast activation protein (FAP) was identified as a new target for CAR T-cells, which appeared to be overexpressed in the tumor area [30]. Potent anti-GBM activity with chlorotoxin-directed CAR T-cells was recently demonstrated, particularly in tumors lacking expression of other GBM-associated antigens [31]. In the murine model, an improved response to therapy with CAR T-cells targeting EGFRvIII with the aid of interleukin 12 (IL-12) was recognized. IL-12 not only increased the cytotoxicity of CAR T-cells but also reformed the immunocompromised microenvironment [32].
To increase the efficacy of CAR T-cells, the inhibition of PP2A with LB-100 was investigated, showing that LB-100 improved anti CAIX CAR T-cell treatment both in vitro and in vivo [33]. PD-1 knockout seems to increase the lytic activity of EGFRvIII-CAR T-cells against PD-L1+ EGFRvIII+ GBM cells [34]. In the context of PD-1 checkpoint blockade, CAR T-cells with PD-1 blockade not only showed higher killing efficiency in vitro, but also resulted in an increased immunological response by tumor infiltrating lymphocytes (TILs) in the mouse model [35]. The problem of the immunosuppressive environment of GBM tumors was addressed by Li et al. in their study on TGFβ-resistant CAR-Ts for GBM therapy. They showed enhanced anti-tumor efficacy of EGFRvIII-specific CAR-T and prolonged survival in mice bearing GBM. Furthermore, a polarizing pro-inflammatory and anti-tumorigenic response was evident by an increased expression of M1 polarization markers [36]. A complication of CAR-T cell therapy is allorejection in the case of a graft-versus-host reaction.
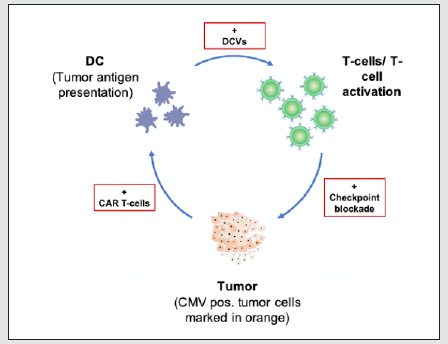
Insufficient specificity of the T-cells from the receptor to the antibodies causes the response also to non-tumoral antibodies, so that a cytokine-release syndrome (CRS) can occur, a systemic inflammatory reaction due to an immune cell activation [37]. Therefore, high specificity must be ensured in the engineering of CAR T-cells. Choe et al. reported controlled tumor cell killing by SynNotch CAR T-cells. In the mouse model, a single intravenous infusion of EGFRvIII synNotch CAR T-cells showed higher antitumor efficacy and T-cell durability than in conventionally expressed CAR T-cells without off-tumor killing [38]. In vitro studies confirm that CMV pp65 specific T-cells are able to kill glioblastoma cells. However, clinical studies further confirming this are lacking. A recent case report demonstrated the response of antitumor therapy of allogenic natural killer cells and pp65 pulsed dendritic cells. After therapy, no residual tumor was seen on imaging [39] (Figure 1).
Figure 1: The cancer immunity cycle. Immunotherapy is used to directly target tumor cells in an individualized manner while concomitantly strengthening the host anti-tumor response and thus developing synergistic effects.
The role of Cytomegalovirus
The role of detection of components of CMV in glioblastoma comes into question as a link to immunotherapy. Krenzlin et al. recently demonstrated in vivo the significant increase of tumor volume with a worse overall survival in CMV infected mice. PDGF as CMV induced factor seemed to play a major role in pericyte recruitment, angiogenesis and tumor growth [27]. A prospective randomized study showed the outcome after vaccination with CMV pp65 RNA loaded dendritic cells. Significant increases in IFNγ+, TNFα+, and CCL3+ polyfunctional, CMV-specific CD8+ T-cells were observed. These results could serve as potential biomarkers for the effective response of immunotherapy [40]. Long-term survival in GBM patients after CMV pp65-targeted vaccination was studied when in addition to temozolomide, 11 patients received three doses of the vaccine, showing an increased ratio and proliferation of regulatory T-cells. The patients showed long-term progression free survival and overall survival [41].
Figure 2: CMV detection in murine GBM cells. GL261-Luc2 murine glioblastoma cells 24 hours (a) and 48 hours (b) after mCMV-eGFP infection. MOI=1. (Einheuser et al, unpublished).
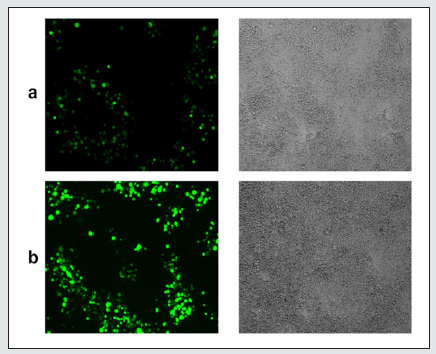
Autologous polyclonal CMV pp65-specif T-cells were studied after administration after manufacture, three weeks of lymphodepletion, and dose-tense temozolomide in a Phase I/II trial. Increased circulating numbers of CMV+ CD8+ T-cells were seen but suppressed effector activity was evident. Furthermore, CMV seropositivity did not guarantee a tumor response to CMVspecific T-cells [42]. In a prospective study with autologous CMVspecific T-cells, twenty-eight patients received adjuvant treatment. The long-term survival of patients treated before tumor recurrence was significantly increased [43]. With regard to the tumor immune microenvironment, it could be shown that a lower number of tumorinfiltrating CD3+ T-cells in recurrent tumors was evident in patients with increased overall survival after T-cell therapy. In addition, these patients showed low to undetectable PD-L1 expression in tumor cells, suggesting that absence of PD-L1 immunosuppression favors effective immune control with adoptive T-cells [44].
Benefits of using single chain T-cell receptors
To reduce or eliminate the probability of graft-versus-host reactions, the specificity of the TCR has to be adjusted. Aggen et al. describe the possibility of a single-chain VαVβ T cell receptor. By combining stabilized Vα/Vβ single-chain TCR (scTv) with intracellular signaling domains such as Lck and CD28, mispairing of exogenous TCR chains with endogenous ones could be prevented, so that a functional T cell response could be activated (19). Additionally, Knies et al. compared gp100(280-288) with p53(264- 272) tumor antigen-specific scTCR with regard to mispairing with TCRα. This showed that mispairing was largely reduced after transduction into human TCRα/β-positive T-cells. The generation of dc/scTCR-modified cytomegalovirus/tumor antigen-bispecific T-cells to enhance T-cell activation in CMV-infected tumor patients. The authors conclude that optimized scTCR/Cα inhibits residual TCR mismatching to achieve safe adoptive immunotherapy for large endogenous TCRα/β-positive T-cells [20].
Conclusions
New approaches in immunotherapy for glioblastoma are lacking. Weaknesses and obstacles need to be overcome in therapy with CAR-T-cells. In this context, single chain TCRs may play an important role. Regarding tumor immunocompromised microenvironment, cytomegalovirus appears to play an essential role in oncomodulation in glioblastoma. This knowledge can be implemented in experimental research to act as a target for potential immunotherapy with CAR T-cells.
List of Abbreviations
GBM: Glioblastoma
PD-1: Programmed death ligand 1
TAA : Tumor associated antigens
CAR: Chimeric antigen receptor
TCR: T-cell receptor
CMV: Cytomegalovirus
scFv: Single chain fragment variable
MGMT: O(6)-methylguanine-DNA-methyltransferase
PD-L1: Programmed cell death ligand 1
DCs: Dendritic cells
EVs: Extracellular vesicles
FAP: Fibroblast activation protein
IL-12: Interleukin 12
PP2A: Protein phosphatase 2
EGFRvIII: Epidermal growth factor receptor variant III
TILs: Tumor infiltrating lymphocytes
CRS: Cytokine-release syndrome
IFNγ: Interferon-γ
TNFα: Tumor necrosis factor α
CCL3: C-C Motif Chemokine Ligand 3
CD8: Cluster of differentiation 8
CD3: Cluster of differentiation 3
Lck: Lymphocyte-specific protein tyrosine kinase
CD28: Cluster of differentiation 28
CAIX: Carbonic anhydrase IX
TGFβ: Transforming Growth Factor β
PDGF: Platelet-derived growth factor
References
- Tamimi AF, Juweid M (2017) Glioblastoma: Epidemiology and Outcome of Glioblastoma. Brisbane, Australia: Codon Publications.
- Liu Y, Tyler E, Lustick M, Klein D, Walter KA (2019) Healthcare Costs for High-grade Glioma. Anticancer Res 39(3): 1375-1381.
- Davis ME (2016) Glioblastoma: Overview of Disease and Treatment. Clin J Oncol Nurs 20(5 Suppl): 2-8.
- Rivera AL, Pelloski CE, Gilbert MR, Colman H, La Cruz C de, et al. (2010) MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol 12(2): 116-121.
- Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, et al. (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344(6190): 1396-1401.
- Da Ros M, Gregorio V de, Iorio AL, Giunti L, Guidi M, et al. (2018) Glioblastoma Chemoresistance: The Double Play by Microenvironment and Blood-Brain Barrier. Int J Mol Sci 19(10): 2879.
- Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, et al. (2018) Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv 4(3): 2766.
- Sena IFG, Paiva AE, Prazeres PHDM, Azevedo PO, Lousado L, et al. (2018) Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med 7(4): 1232-1239.
- Woroniecka K, Fecci PE (2018) T-cell exhaustion in glioblastoma. Oncotarget 9(82): 35287-35288.
- Wilcox JA, Ramakrishna R, Magge R (2018) Immunotherapy in Glioblastoma. World Neurosurg 116: 518-528.
- Choi BD, Maus MV, June CH, Sampson JH (2019) Immunotherapy for Glioblastoma: Adoptive T-cell Strategies. Clin Cancer Res 25(7): 2042-2048.
- Bagley SJ, Desai AS, Linette GP, June CH, O Rourke DM (2018) CAR T-cell therapy for glioblastoma: Recent clinical advances and future challenges. Neuro Oncol 20(11):1429-1438.
- Chuntova P, Downey KM, Hegde B, Almeida ND, Okada H (2018) Genetically Engineered T-Cells for Malignant Glioma: Overcoming the Barriers to Effective Immunotherapy. Front Immunol 9: 3062.
- Frey NV (2019) Chimeric antigen receptor T cells for acute lymphoblastic leukemia. Am J Hematol 94(1): 24-27.
- Mato AR, Thompson MC, Nabhan C, Svoboda J, Schuster SJ (2017) Chimeric Antigen Receptor T-Cell Therapy for Chronic Lymphocytic Leukemia: A Narrative Review. Clin Lymphoma Myeloma Leuk 17(12): 852-856.
- Chavez JC, Bachmeier C, Kharfan Dabaja MA (2019) CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol 10:2040620719841581.
- Mikkilineni L, Kochenderfer JN (2021) CAR T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol 18(2): 71-84.
- Yang Y, Jacoby E, Fry TJ (2015) Challenges and opportunities of allogeneic donor-derived CAR T cells. Curr Opin Hematol 22(6): 509-515.
- Aggen DH, Chervin AS, Schmitt TM, Engels B, Stone JD, et al. (2012) Single-chain VαVβ T-cell receptors function without mispairing with endogenous TCR chains. Gene Ther 19(4): 365-374.
- Knies D, Klobuch S, Xue SA, Birtel M, Echchannaoui H, et al. (2016) An optimized single chain TCR scaffold relying on the assembly with the native CD3-complex prevents residual mispairing with endogenous TCRs in human T-cells. Oncotarget 7(16): 21199–21221.
- Al Mana H, Yassine HM, Younes NN, Al-Mohannadi A, Al-Sadeq DW, et al. (2019) The Current Status of Cytomegalovirus (CMV) Prevalence in the MENA Region: A Systematic Review. Pathogens 8(4): 213.
- Cannon MJ, Schmid DS, Hyde TB (2010) Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 20(4): 202-213.
- Bai B, Wang X, Chen E, Zhu H (2016) Human cytomegalovirus infection and colorectal cancer risk: A meta-analysis. Oncotarget 7(47): 76735-76742.
- Samanta M, Harkins L, Klemm K, Britt WJ, Cobbs CS (2003) High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J Urol 170(3): 998-1002.
- Rahman M, Dastmalchi F, Karachi A, Mitchell D (2019) The role of CMV in glioblastoma and implications for immunotherapeutic strategies. Oncoimmunology 8(1): 1514921.
- Dziurzynski K, Chang SM, Heimberger AB, Kalejta RF, McGregor Dallas SR, et al. (2012) Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol 14(3): 246-255.
- Krenzlin H, Behera P, Lorenz V, Passaro C, Zdioruk M, et al. (2019) Cytomegalovirus promotes murine glioblastoma growth via pericyte recruitment and angiogenesis. J Clin Invest 129(4): 1671-1683.
- Halford Z, Anderson MK, Bennett LL, Moody J (2021) Tisagenlecleucel in Acute Lymphoblastic Leukemia: A Review of the Literature and Practical Considerations. Ann Pharmacother 55(4): 466-479.
- Martínez Bedoya D, Dutoit V, Migliorini D (2021) Allogeneic CAR T Cells: An Alternative to Overcome Challenges of CAR T Cell Therapy in Glioblastoma. Front Immunol 12: 640082.
- Ebert LM, Yu W, Gargett T, Toubia J, Kollis PM, et al. (2020) Endothelial, pericyte and tumor cell expression in glioblastoma identifies fibroblast activation protein (FAP) as an excellent target for immunotherapy. Clin Transl Immunology 9(10): 1191.
- Wang D, Starr R, Chang WC, Aguilar B, Alizadeh D, et al. (2020) Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci Transl Med 12(533).
- Agliardi G, Liuzzi AR, Hotblack A, Feo D de, Núñez N, et al. (2021) Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun 12(1): 444.
- Cui J, Wang H, Medina R, Zhang Q, Xu C, et al. (2020) Inhibition of PP2A with LB-100 Enhances Efficacy of CAR-T Cell Therapy Against Glioblastoma. Cancers (Basel) 12(1): 139.
- Zhu H, You Y, Shen Z, Shi L (2020) EGFRvIII-CAR-T Cells with PD-1 Knockout Have Improved Anti-Glioma Activity. Pathology oncology research 26(4): 2135-2141.
- Song Y, Liu Q, Zuo T, Wei G, Jiao S (2020) Combined antitumor effects of anti-EGFR variant III CAR-T cell therapy and PD-1 checkpoint blockade on glioblastoma in mouse model. Cell Immunol 352: 104112.
- Li Y, Wu H, Chen G, Wei X, Wang C, Zhou S et al. (2020) Arming Anti-EGFRvIII CAR-T With TGFβ Trap Improves Antitumor Efficacy in Glioma Mouse Models. Front Oncol 10:1117.
- Land CA, Musich PR, Haydar D, Krenciute G, Xie Q (2020) Chimeric antigen receptor T-cell therapy in glioblastoma: charging the T cells to fight. J Transl Med 18(1): 428.
- Choe JH, Watchmaker PB, Simic MS, Gilbert RD, Li AW, et al. (2021) SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med 13(591).
- Gumrukcu S, Nguyen TX, White RL, Howell GT, Musikanth P (2021) Allogeneic Natural Killer and Cytomegalovirus (CMV)-pp65 Pulsed Dendritic Cells Induced Complete Response Through 15 Months in a Patient with Recurrent Glioblastoma: A Case Study. Am J Case Rep 22: 931030.
- Reap EA, Suryadevara CM, Batich KA, Sanchez Perez L, Archer GE, et al. (2018) Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma. Cancer Res 78(1): 256-264.
- Batich KA, Reap EA, Archer GE, Sanchez Perez L, Nair SK, et al. (2017) Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin Cancer Res 23(8):1898-1909.
- Weathers SP, Penas Prado M, Pei BL, Ling X, Kassab C, et al. (2020) Glioblastoma-mediated Immune Dysfunction Limits CMV-specific T Cells and Therapeutic Responses: Results from a Phase I/II Trial. Clin Cancer Res 26(14): 3565-3577.
- Smith C, Lineburg KE, Martins JP, Ambalathingal GR, Neller MA, et al. (2020) Autologous CMV-specific T cells are a safe adjuvant immunotherapy for primary glioblastoma multiforme. J Clin Invest 130(11): 6041-6053.
- Walker DG, Shakya R, Morrison B, Neller MA, Matthews KK, et al. (2019) Impact of pre-therapy glioblastoma multiforme microenvironment on clinical response to autologous CMV-specific T-cell therapy. Clin Transl Immunology 8(11): 01088.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




