
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Case Report(ISSN: 2637-6628) 
Solitary Fibrous Tumor of the Pineal Region: Report of a Rare Case in Unusual Location Volume 6 - Issue 2
Samasuk Thammachantha1*, Sirirat Khunvutthidee2 and Kullapat Veerasarn3
- 1Department of Pathology, Neurological Institute of Thailand (NIT), Thailand
- 2Department of Radiology, Neurological Institute of Thailand (NIT), Thailand
- 3Department of Neurosurgery, Neurological Institute of Thailand (NIT), Thailand
Received: March 24, 2022; Published: April 05, 2022
Corresponding author: Samasuk Thammachantha, Department of Pathology, Neurological Institute of Thailand (NIT), 312 Ratchawithi Rd, Thung-Phayathai, Rajthewi, Bangkok, Thailand 10400.
DOI: 10.32474/OJNBD.2022.06.000235
Abstract
A 55-year-old female was admitted with history of ataxia. Magnetic resonance imaging (MRI) imaging reveled pineal region mass. Histology revealed thin-walled branching vessels of tumor with STAT6 positivity. Here we report a case of pineal solitary fibrous tumor, as well as updated current nomenclature and risk assessment.
Keywords: Solitary Fibrous Tumor; Pineal Region; STAT6
Introduction
Intracranial solitary fibrous tumor (SFT) is a rare non-meningothelial mesenchymal tumor, accounting for 0.4% of all intracranial tumors [1,2]. Primary intracranial SFT in the pineal region has been rarely reported. Here we report a rare case of the pineal region SFT.
Case Report
Figure 1: Horizontal section of pineal mass with isointensity on T1W MRI (A), iso- to hypointense on T2W MRI (B). Homogeneous contrast enhancement of the mass on postcontrast T1W MRI; sagittal section (C) and coronal section (D).
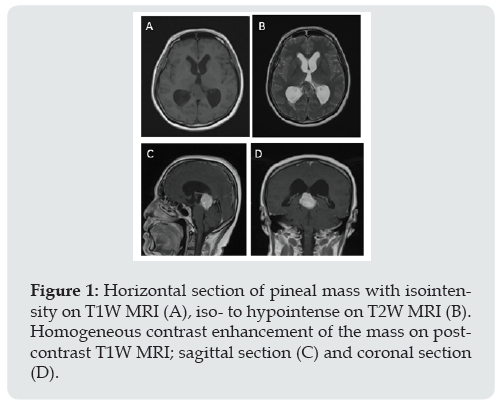
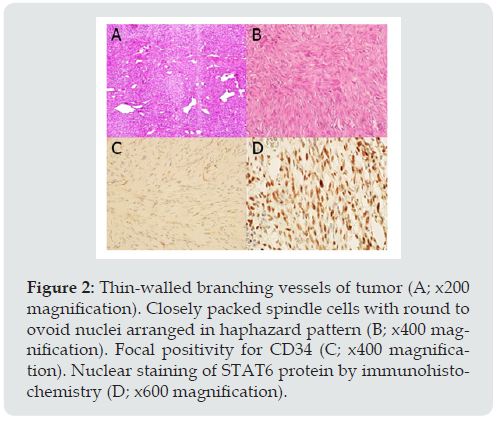
A 55-year-old female presented with one-month history of ataxia. Neurological examination revealed full motor power and intact sensory system. Absence of abnormal reflex. Cranial nerve and other physical examination were not remarkable. Magnetic resonance imaging (MRI) showed a lobulated enhancing pineal mass with tectal plate compression, produced hydrocephalus. T2-weighted image revealed iso-to hypointensity of the mass with homogeneous contrast enhancement (Figure 1). The mass was found to be attached to the tentorium cerebelli. Pineal meningioma, pineal parenchymal tumor and germ cell tumors were in the differentials. Surgical resection was performed in prone position via right occipital transtentorial approach. The tumor appeared as a grayish white, firm, well-defined and highly vascularized mass. Subtotal resection was achieved and confirmed by post-operative MRI. Microscopic examination (Figure 2) revealed haphazard pattern of the tumor. Tumor cells were round to spindle in shape, accompanied with dilated branching vessels (staghorn appearance). Immunohistochemical study exhibited focal and weak immunore activity for CD34, diffuse and strong immunoreactivity for STAT6, but negative for Epithelial membrane antigen(EMA), Somatostatin receptor-2a(SSTR-2a), Glial fibrillary acidic protein(GFAP), Synaptophysin, S-100, Smooth muscle actin and Desmin. No mitotic figure or necrosis was found. Proliferative index (Ki-67) was less than 1%. The patient developed left hemiparesis and alteration of consciousness at post-operative day [2]. Computed tomography (CT) scan have shown hypodensity lesion at right thalamus, suspected of right thalamic infarction. Post-operative MRI demonstrated partial tumor removal and ischemic change of right thalamus and posterior limb of internal capsule. On 1 year follow up, patient was doing well.
Figure 2: Thin-walled branching vessels of tumor (A; x200 magnification). Closely packed spindle cells with round to ovoid nuclei arranged in haphazard pattern (B; x400 magnification). Focal positivity for CD34 (C; x400 magnification). Nuclear staining of STAT6 protein by immunohistochemistry (D; x600 magnification).
Discussion
SFTs are rare vascular tumors which can occurs any part of the body, most commonly seen in the lower extremities, pelvis, head and neck areas [3]. Pineal region location is rare with very few reported cases in PMC literatures. Because SFTs are remarkably similar to meningioma in clinical and radiographic presentation, histological confirmation is the only tool of distinguishing the diagnosis. Histologic features of this tumor are “patternless”, marked by area of dense hyalinization, and fibroblasts permeate between collagen bundles. Many subsets of this tumor are reported, some with adipocytic component. Currently, there are several immunohistochemical stains have been developed, especially the important one; STAT6. Gene fusion of NAB2 and STAT6 results in nuclear localization of STAT6 protein. It is detected with 97% both sensitivity and specificity by immunohistochemistry [4,5]. In 2021 WHO Classification of Tumors of the Central Nervous System (5th edition), the term “hemangiopericytoma” was removed, only “Solitary fibrous tumor” has been used (rather than the hybrid term of both). This nomenclature change aligns with soft tissue pathology nomenclature [6]. Biologically, it was clearly fibroblast rather than pericyte [7]. Surgical resection is the treatment of first choice for CNS SFT [8]. Complete resection is preferred and considered as an effective approach to reduce local recurrence and metastasis [9,10]. According to the 2017 risk stratification criteria by Demicco et al, Risk stratification models have replaced the use of ambiguous nomenclature like “benign SFT” and “malignant SFT” [11] (Table 1). In conclusion, SFTs are rare intracranial tumors arising from pericytes. We have reported a rare case of SFTs that arose in the pineal region. Correct diagnosis of SFTs can only be made through immunohistochemical analysis because of the clinical and radiological similarity between the SFTs and the meningiomas. In particular, the unusual location of SFT often makes it difficult to diagnose via radiological study alone, due to the radiological features are not pathognomonic. Biologically, SFTs are more invasive than meningiomas. So, it is very important to make a correct diagnosis. Pathologists must be aware of SFT in this unusual location.
References
- Rutkowski MJ, Jian BJ, Bloch O, Cheng Chen, Michael E Sughrue, Tarik Tihan, et al. (2012) Intracranial hemangiopericytoma: Clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer 118(6): 1628-1636.
- Schiariti M, Goetz P, El-Maghraby H, Jignesh Tailor, et al. (2011) Hemangiopericytoma: Long term outcome revisited. Clinical article. J Neurosurg 114(3): 747-755.
- Giannini C, Rushing EJ, Hainfellner JA (2007) Hemangiopericytoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. Lyon: International Agency for Research of Cancer pp. 178-180.
- Schweizer L, Koelsche C, Sahm F, Rosario M Piro, David Capper, et al. (2013) Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol 125(5): 651-658.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2016) World HealthOrganization Classification of Tumours of the Central Nervous System. 4th edition, updated ed. Lyon, International Agency for Research on Cancer.
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Cynthia Hawkins, et al. (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol 23(8): 1231-1251.
- Gengler C, Guillou L (2006) Solitary fibrous tumour and haemangiopericytoma: Evolution of a concept. Histopathology 48(1): 63-74.
- Chen H, Zeng XW, Wu JS, Ya-Fang Dou, Yin Wang, et al. (2012) Solitary fibrous tumor of the central nervous system: a clinicopathologic study of 24 cases. Acta Neurochir (Wien) 154: 237-248.
- Damodaran O, Robbins P, Knuckey N, Michael Bynevelt, George Wong, et al. (2014) Primary intracranial haemangiopericytoma: Comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci 21: 1310-1314.
- Metellus P, Bouvier C, Guyotat J, Stéphane Fuentes, Anne Jouvet, et al. (2007) Solitary fibrous tumors of the central nervous system: clinicopathological and therapeutic considerations of 18 cases. Neurosurgery 60(4): 715-722.
- Demicco E, Wagner M, Maki R, Vishal Gupta, Ilya Iofin, et al. (2017) Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol 30: 1433-1442.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





