
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Mini Review(ISSN: 2637-6628) 
Neuroinflammation in Alzheimer’s Disease: Current Therapeutic Approaches and Recent Progress Volume 4 - Issue 5
Rohit Shukla1 and Tiratha Raj Singh1,2*
- 1Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology (JUIT), Waknaghat, Solan, Himachal Pradesh, India
- 2Centre for Excellence in Healthcare Technologies and Informatics (CEHTI), Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology, India
Received: November 24, 2020; Published:December 09, 2020
Corresponding author: Tiratha Raj Singh, Associate Professor, Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology, Waknaghat, Solan, 173215, Himachal Pradesh, India
DOI: 10.32474/OJNBD.2020.04.000200
Abstract
Alzheimer’s Disease (AD) is a progressive neurological disorder, characterized by amyloid-beta (Aβ) and neurofibrillary tangles (NFTs). Recently a new factor called neuroinflammation is added which can directly play a key role in the progression of AD. The recent studies in humans and mice had shown that aggregated protein (Aβ and NFTs) binds to the microglia cells (the resident innate immune cells) found in the central nervous system and activates the innate immune response which is characterized by the release of inflammatory mediators and increase the disease progression through the neuroinflammation. Several factors such as traumatic brain injury, obesity, etc. can play a major role in neuroinflammation. Hence targeting the immune mechanism and reduce the risk factors could lead to future promising therapeutics for AD.
Keywords: Alzheimer’s Disease; Neurofibrillary Tangles; Drug Development; Microglia; Neuroinflammation
Introduction
In the first glimpse, immunology and neurobiology are not very much different. Based on the cellular perspective, the brain represents the stasis mechanism while the motion mechanism is represented by the immune system. We can combine these two mechanisms to understand neurodegenerative diseases such as AD, Parkinson’s disease, etc. The recent evidence suggested that inflammation is playing a key role in disease progression. Hence understanding the mechanism of the nervous system and immune system interaction can prevent or hinder the progression of CNS diseases. In AD, the neuroinflammation is not only activated by the Aβ and NFTs formation however it also participates in the disease pathogenesis even more than Aβ and NFTs [1]. Several key genes like TREM2 [2] and CD33 [3] play a key role in the neuroinflammation induced AD progression. The analysis of several stages of dementia such as mild cognitive impairment showed the early and significant participation of inflammation in the AD progression [4]. In this review, we will describe the various cell types which are involved in neuroinflammation along with the therapeutic approaches and ongoing clinical trials against AD neuroinflammation.
Neuroinflammation in Alzheimer’s Disease
Other than two major hallmarks such as Aβ and NFTs in AD, neuroinflammation is the third core neuropathological feature [5]. Around the NFTs and plaques, the activated astrocytes and microglia cells are also found. In AD brains the inflammatory markers or pro-inflammatory cytokines level is also increased [6]. This evidence of inflammatory reactions suggests the response of the immune system against the Aβ and NFTs deposition [7]. Ultimately the neuronal damage or neuron cell death happens due to the uncontrolled activation of these inflammatory processes through microglia and other cells which is very harmful and increases the AD progression [8] (Figure 1). In this section, we will discuss the different type of cells which participates in the neuroinflammation mechanism.
Figure 1: The difference in the microglia functions under physiological and pathological conditions.
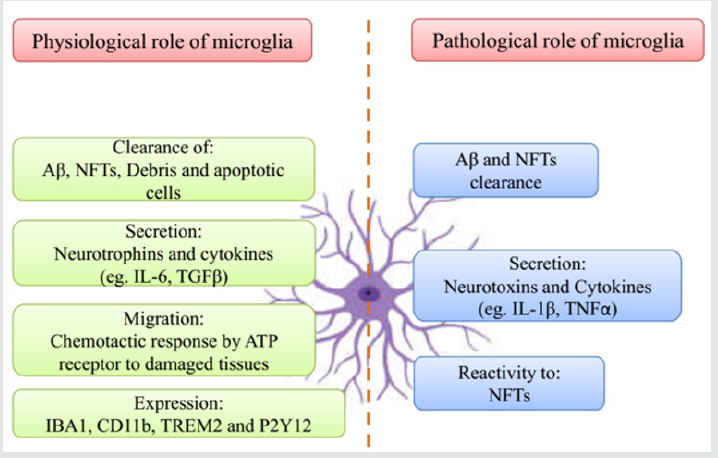
Microglia
Microglia is a type of resident immune cells located in the central nervous system (CNS). They perform several functions such as antigen-presenting, phagocytosis, and produce immune mediators like peripheral monocytes. The microglia cells interact with the Aβ and NFTs with the help of G-protein coupled receptors (CMKLR1 and FPR2), TERM2, scavenger receptors (MARCO, CD36, SCARA-1, SCARB-1, and RAGE), toll-like receptors (TLR9, TLR6, TLR4, and TLR2), CD47 and α6β1 integrin [9]. The microglia cells are activated by using the NF-κB pathway after the initial recognition [10]. Due to the constant presence of the senile plaques and Aβ, the microglia cells are continuously activated. Due to the pro-inflammatory mediators which are produced by Aβ due to the chronically activated microglia, the phagocytosis ability is decreased, and which results in prolonged neuroinflammation in AD [11].
Astrocytes
Astrocytes are also a type of glial cells that participates in the waste clearance, nutritional supplementation to neurons, and maintenance of the blood-brain barrier. The increasing glial fibrillary acidic protein (GFAP) indicates astrocyte activation [12], astrogliosis, and astrocyte atrophy [13] which happens before the AD even before the Aβ and NFTs formation. The astrocytes can also be activated by the NF-κB pathway [14]. In some cases, these activated astrocytes destruct the Aβ itself, and use the ApoE lipidation [15] to increase the phagocytosis of microglia. Although these activated astrocytes increase the neuroinflammation through the inflammatory mediators [16].
The oligodendrocytes are the key resource of the myelin in the central nervous system,. The oligodendrocytes role in AD is still a mystery and scientists are working on it. A recent study showed that oligodendrocytes can contribute towards the neuroinflammation process because they can do the complement system synthesis [17].
Cytokines
In AD, cytokines play a diverse role in neuroinflammation. The production of Aβ is increased through the TNF-α by β- and γ-secretase through the amyloid precursor protein (APP) [18]. The same function (generation of Aβ) is performed by IL-1 also [18]. By using the p38-MAPK pathway the tau protein phosphorylation is also increased by the IL-1 [19]. The IL-6 also increases the tau protein phosphorylation by using the cdk5/p35 pathway [20] along with the higher expression of APP [21]. In the CNS, the microglial migration is regulated by chemokines. In the human mononuclear cells, the Aβ increased the production of CCL2, CCL3, CCL4, and CXCR8 [22]. For the synapse maturation, function and for maintaining the microglial resting-state maintenance the CX3CR1/CX3CL1 play an important role [23]. The CX3CR1/CX3CL1 is responsible for the cognitive decline and amyloid deposition is shown in an animal model recently [24]. The neurons, astrocytes, microglia, and oligodendrocytes produce the components of the complement system in the CNS [25]. The role of the complement system in AD is still unexplored very well. Due to the interaction of C1q with the Aβ, the alternate complement pathway is activated, and it induces the neuroinflammation and phagocytosis of Aβ [26]. The C3 deficiency is associated with amyloid phagocytosis and higher Aβ accumulation in an animal model. Association between the complement inhibitor, apolipoprotein J, and complement receptor 1 indicates the potential role of the complement system to AD in a genome-wide association study [27].
Potential Therapeutics for Neuroinflammation in AD
The current treatment of AD can provide only symptomatic
relaxation instead of a permanent cure. There are two types of
medications available to treat AD. First are the cholinesterase
inhibitors (rivastigmine, donepezil, and galantamine) and
second are the NMDA inhibitors such as memantine. There is an
urgent need to find an alternate therapeutic approach due to the
higher failure rate in AD drug discovery [28]. Hence targeting
the neuroinflammation mechanism by using several therapeutic
strategies can be beneficial for AD management.
Several approaches are developed such as inhibiting cytokines
release, reducing cytokines gene expression, and preventing
cytokines binding to their receptors [29]. The minocycline is a type
of anti-inflammatory molecule shown a reduction in the release of
the cytokines from the astrocytes and hence it showed the benefits
in the cognitive deficits in AD mouse models [30,31]. In another
study, it was found that inhibition of tau phosphorylation kinases
can also give the suitable results of modulating neuroinflammation
in animal models [32]. The reduction in oxidative injury also
showed a promising result. Like inducible nitric oxide synthase and
cyclooxygenase-2 inhibition showed good results in the in vitro and
in vivo animal studies [33]. Although the Dimebon (latrepirdine)
acts as a mitochondrial enhancer and failed in a clinical trial (NCT
00912288) [34]. It showed that more efforts should be made to find
the therapeutics for restoring the mitochondrial dysfunction which
can help in the synaptic and neuronal function restore mechanism
[35].
In other approaches, the microglial function is targeted through
experimental manipulations to reduce the axonal dystrophy and
increase the microglial encapsulation of amyloid plaques [36]. The
recent studies showed that anti-Aβ antibody [36] or anti-ApoE
antibody passive immunization promotes the microglia recruitment
in the region of amyloid plaques in AD mouse models. It is shown
that knockout of CX3CR1 (chemokine receptor) in microglia can
reduce the plaque burden [37] by increasing the formation of
microglial barrier in the region of amyloid plaques [36]. The small
molecules such as AZD8797 [38] and CX3CR1 and its receptor
targeting antibody can also neutralize the CX3CR1 function.
Although the CX3CR1 cannot target without concerns [39].
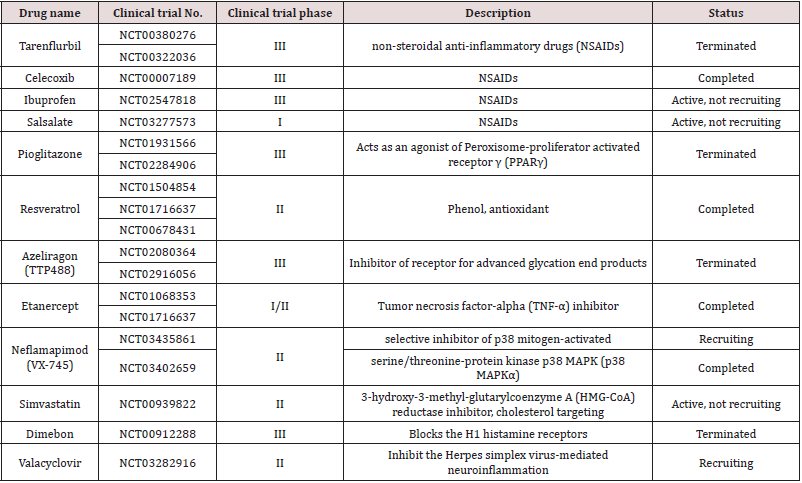
There are many other mechanisms by which the inhibitors or
modulator can be designed. Several clinical trial studies are going
on to find the potential inhibitor against AD [40] and we have
summarized a few of them in (Table 1).
Table 1 The table describes the different type of neuroinflammatory drugs which are in the clinical trial.
Conclusion and Future Perspective
AD is a complex, multifactorial disease and cannot be explained by only one hypothesis. On dated 22/11/2020, the 2453 clinical trials are listed on the clinicaltrials.gov website for AD including drugs, therapies, and imaging markers. To date, there is no drug that can completely cure AD due to the higher failure rate in the late clinical trials. Lesions from the previously failed clinical trials suggest that the pathophysiological mechanisms are not only limited to the amyloid and tau hypotheses while other mechanisms such as neuroinflammation and others are also involved in the disease pathology progression. The CNS innate immune response triggered neuroinflammation plays a key role in the disease pathogenesis. Additionally, neuroinflammation is an early cause of AD instead of a late consequence of AD. Due to the key role of neuroinflammation in AD the anti-inflammatory therapeutic development which can improve the function of microglia can be a promising therapeutic strategy to alter the progression of AD. We believe that the drugs which can act as the anti-inflammatory agent can be beneficial for AD and several other neurodegenerative diseases.
Acknowledgement
Authors acknowledge the ICMR grant (ISRM/11(53)/2019) for providing the Senior Research Fellowship to RS.
References
- Bin Zhang, Chris Gaiteri, Liviu-Gabriel Bodea, Zhi Wang, Joshua McElwee, et al. (2013) Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153(3): 707-720.
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, et al. (2013) TREM2 variants in Alzheimer’s disease. The New England Journal of Medicine 368(2): 117-127.
- Griciuc A, Serrano-Pozo A, Parrado A R, Lesinski A N, Asselin C N, et al. (2013) Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78(4): 631-643.
- Heneka M T, Carson M J, El Khoury J, Landreth G E, Brosseron F, et al. (2015) Neuroinflammation in Alzheimer’s Disease. The Lancet Neurology 14(4): 388-405.
- Pi-Shan Sung, Po-Yu Lin, Chi-Hung Liu, Hui-Chen Su, Kuen-Jer Tsai (2020) Neuroinflammation and Neurogenesis in Alzheimer’s Disease and Potential Therapeutic Approaches. International Journal of Molecular Sciences 21(3): 701.
- Robert E Mrak, W Sue T Griffin (2005) Glia and their cytokines in progression of neurodegeneration. Neurobiology of Aging 26(3): 349-354.
- Seung Hyun Kim, Min Young Noh, Hee-Jin Kim, Ki-Wook Oh, Jinseok Park, et al. (2019) A Therapeutic Strategy for Alzheimer’s Disease Focused on Immune-inflammatory Modulation. Dementia and Neurocognitive Disorders, 18(2): 33-46.
- Webers A, Heneka M T, Gleeson P A (2020) The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunology and Cell Biology 98(1): 28-41.
- Yang Yu, Richard D Ye (2015) Microglial Aβ receptors in Alzheimer’s disease. Cellular and Molecular Neurobiology 35(1): 71-83.
- C K Combs, J C Karlo, S C Kao, G E Landreth (2001) beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. The Journal of Neuroscience 21(4): 1179-1188.
- Hickman S E, Allison E K, El Khoury J (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 28(33): 8354-8360.
- Markus P Kummer, Thea Hammerschmidt, Ana Martinez, Dick Terwel, Gregor Eichele, et al. (2014) Ear2 Deletion Causes Early Memory and Learning Deficits in APP/PS1 Mice. The Journal of Neuroscience 34(26): 8845-8854.
- Chia-Yu Yeh, Bhamini Vadhwana, Alexei Verkhratsky, José J Rodríguez (2011) Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer’s disease. ASN neuro 3(5): 271-279.
- Carrero I, Gonzalo M R, Martin B, Sanz-Anquela J M, Arévalo-Serrano J, et al. (2012) Oligomers of β-amyloid protein (Aβ1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1β, tumour necrosis factor-α, and a nuclear factor κ-B mechanism in the rat brain. Experimental Neurology 236(2): 215-227.
- Terwel D, Steffensen K R, Verghese P B, Kummer M P, Gustafsson J-Å, et al. (2011) Critical role of astroglial apolipoprotein E and liver X receptor-α expression for microglial Aβ The Journal of Neuroscience 31(19): 7049-7059.
- Lian H, Litvinchuk A, Chiang A C-A, Aithmitti N, Jankowsky J L, et al. (2016) Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. The Journal of Neuroscience 36(2): 577-589.
- Hosokawa M, Klegeris A, Maguire J, McGeer P L (2003) Expression of complement messenger RNAs and proteins by human oligodendroglial cells. Glia 42(4): 417-423.
- Yung-Feng Liao, Bo-Jeng Wang, Hui-Ting Cheng, Lan-Hsin Kuo, Michael S Wolfe (2004) Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. The Journal of Biological Chemistry 279(47): 49523-49532.
- Yuekui Li, Ling Liu, Steven W Barger, W Sue T Griffin (2003) Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. The Journal of Neuroscience 23(5): 1605-1611.
- Rodrigo A Quintanilla, Daniel I Orellana, Christian González-Billault, Ricardo B Maccioni (2004) Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Experimental Cell Research 295(1): 245-257.
- Ringheim G E, Szczepanik A M, Petko W, Burgher K L, Zhu S Z, et al. (1998) Enhancement of beta-amyloid precursor protein transcription and expression by the soluble interleukin-6 receptor/interleukin-6 complex. Brain Research. Molecular Brain Research 55(1): 35-44.
- Hessel A Smits, Annemarie Rijsmus, Joyce H van Loon, Jesse WY Wat, Jan Verhoef, et al. (2002) Amyloid-beta-induced chemokine production in primary human macrophages and astrocytes. Journal of Neuroimmunology 127(1-2): 160-168.
- Rosa C Paolicelli, Giulia Bolasco, Francesca Pagani, Laura Maggi, Maria Scianni, et al. (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333(6048): 1456-1458.
- Sungho Lee, Nicholas H Varvel, Megan E Konerth, Guixiang Xu, Astrid E Cardona, et al. (2010) CX3CR1 Deficiency Alters Microglial Activation and Reduces Beta-Amyloid Deposition in Two Alzheimer’s Disease Mouse Models. The American Journal of Pathology 177(5): 2549-2562.
- Veerhuis R, Nielsen H M, Tenner A J (2011) Complement in the brain. Molecular Immunology 48(14): 1592-1603.
- S D Webster, A J Yang, L Margol, W Garzon-Rodriguez, C G Glabe, et al. (2000) Complement component C1q modulates the phagocytosis of Abeta by microglia. Experimental Neurology 161(1): 127-138.
- Jean Charles Lambert, Simon Heath, Gael Even, Dominique Campion, Kristel Sleegers, et al. (2009) Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nature Genetics 41(10): 1094-1099.
- Jeffrey L Cummings, Travis Morstorf, Kate Zhong (2014) Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Research & Therapy 6(4): 37.
- Rommy von Bernhardi , Cornejo F, Parada G E, Eugenín J (2015) Role of TGFβ signaling in the pathogenesis of Alzheimer’s disease. Frontiers in Cellular Neuroscience 9: 426.
- Claire J Garwood, Jonathan D Cooper, Diane P Hanger, Wendy Noble (2010) Anti-Inflammatory Impact of Minocycline in a Mouse Model of Tauopathy. Frontiers in Psychiatry 1: 36.
- Anna Parachikova, Vitaly Vasilevko, David H Cribbs, Frank M LaFerla, Kim N Green (2010) Reductions in amyloid-beta-derived neuroinflammation, with minocycline, restore cognition but do not significantly affect tau hyperphosphorylation. Journal of Alzheimer’s disease 21(2): 527-542.
- Green H F, Nolan Y M (2012) GSK-3 mediates the release of IL-1β, TNF-α and IL-10 from cortical glia. Neurochemistry International 61(5): 666-671.
- Malinski T (2007) Nitric oxide and nitroxidative stress in Alzheimer’s disease. Journal of Alzheimer’s disease 11(2): 207-218.
- Russell H Swerdlow, Jeffrey M Burns, Shaharyar M Khan (2014) The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochimica Et Biophysica Acta 1842(8): 1219-1231.
- Shevtsova E F, Vinogradova D V, Kireeva E G, Reddy V P, Aliev G, et al. (2014) Dimebon attenuates the Aβ-induced mitochondrial permeabilization. Current Alzheimer Research 11(5): 422-429.
- Carlo Condello, Peng Yuan, Aaron Schain, Jaime Grutzendler (2015) Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nature Communications 6: 6176.
- Zhiqiang Liu, Carlo Condello, Aaron Schain, Roa Harb, Jaime Grutzendler (2010) CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. The Journal of Neuroscience 30(50): 17091-17101.
- Linda Cederblad, Birgitta Rosengren, Erik Ryberg, Nils-Olov Hermansson (2016) AZD8797 is an allosteric non-competitive modulator of the human CX3CR1 receptor. The Biochemical Journal 473(5): 641-649.
- Cao, Jiqing Hou, Jianwei Ping, Jing Cai, Dongming (2018) Advances in developing novel therapeutic strategies for Alzheimer’s disease. Molecular Neurodegeneration 13(1): 64.
- Yuan Dong, Xiaoheng Li, Jinbo Cheng, Lin Hou (2019) Drug Development for Alzheimer’s Disease: Microglia Induced Neuroinflammation as a Target? International Journal of Molecular Sciences 20(3): 558.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




