Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Research Article(ISSN: 2637-6628) 
Impact of the Publication of Chinese Treatment Guideline on the Initial Therapy in Parkinson’s Disease in Beijing Volume 3 - Issue 1
Haitian Nan, Kai Li, Shuhua Li, Wen Su and Haibo Chen*
- Department of Neurology, Beijing Hospital, China
Received:July 31, 2019 ; Published: August 08, 2019
Corresponding author: Haibo Chen, Department of Neurology, Beijing Hospital, China
DOI: 10.32474/OJNBD.2019.02.000153
Abstract
Background: The Guideline for Management of Parkinson’s Disease in China was published in 2006 to standardize Parkinson’s disease treatment. Our objective was to compare the initial PD treatment and their accordance with the recommendations before and after the guideline publication.
Methods: We identified 136 PD patients as part of a hospital-based study in Beijing, and compared the prescriptions of Dopamine Agonists (DA) and levodopa (LD) as initial therapy to evaluate the impact of the publication of Chinese guideline on the therapy of PD.
Results: We found that the publication of the guideline resulted in no difference in initial treatment of PD patients > 65 years. In patients < 65 years, the prescription of DA was significantly increased after the publication of the guideline. There were no significant differences in initial treatment between patients treated in hospitals of different levels or patients with different types of insurance.
Conclusion: The guideline promoted DA utilization as initial treatment in young patients.
Keywords: Parkinson Disease; Initial Therapy; Treatment Guideline; Dopamine Agonist; Beijing
Introduction
The most commonly used antiparkinsonian drugs in China include: anticholinergics (trihexyphenidyl), Amantadine, Levodopa (LD), Dopamine Agonists (DA), Monoamine Oxidase (MAO)-B inhibitors, Catechol-O-Methyltransferase (COMT) inhibitors. LD is the most effective antiparkinsonian drug [1], but its long-term use is associated with motor complications [2]. DA poses less risk of long-term motor complications than LD [3] but is less effective. The challenge during initial PD stages is to control motor symptoms and restore quality of life without increasing the risk of long-term complications. In July 2006, the Guideline for Management of Parkinson’s Disease in China was published. Our objective was to compare the initial PD treatment and their accordance with the recommendations before and after the guideline’s publication.
Subjects and Methods
Participants
One hundred and thirty-six PD patients were enrolled from two hospitals through out-patient department of neurology: Beijing Hospital of the Ministry of Health (class 3-A hospital) and Pinggu traditional Chinese medical hospital (class 2-A hospital). Patients were examined by movement disorders specialists and inquired about the date of symptoms onset, initial treatment, and age at treatment initiation. They were invited to provide all their PD drug prescriptions. Diagnosis was established using standardized criteria [4]. All subjects had at least one prescription of LD, COMT inhibitor (entacapone), Amantadine, anticholinergics (trihexyphenidyl), DA (pramipexole and piribedil), or MAO-B inhibitors (selegiline and rasagiline). Additional inclusion criteria were: disease duration>1 month and Hoehn-Yahr stage less than II.
Chinese Guideline on the Therapy of Pd
The guideline on PD treatment in China was proposed by the Chinese Movement Disorders and Parkinson’s Disease Society. The experts in the study group are all well-recognized on treating PD and related motor disorders. The guideline is a result of a comprehensive review of existing literature and the actual situation in China. The first edition was published in June 2006 in Chinese Journal of Neurology which aimed at making recommendations regarding PD diagnosis and treatment and is widely accessed by both clinical physicians and scientific researchers [5]. The published guideline includes the time of drug utilization and the choice of initial medications in the treatment of early symptoms of Parkinson’s disease. And it was the first time in China to recommend standard Parkinson’s disease treatment. The guideline suggests that DA is the mainstay choice followed by selegiline with or without vitamin E for patients younger than age 65 years without cognitive impairment. When the medications mentioned above fail to improve the symptoms, a combination therapy with compound LD and COMT inhibitor is required. However, for some patients with diminished cognitive function or needing remarkable improvement, compound LD can be the initial therapy. For old (≥65 years) patients, compound LD should be the initial choice, if needed, it can be combined with DA, MAO-B inhibitor or COMT inhibitor. In 2001, America published the third edition of the guidelines for the treatment of PD [6] with detailed description of initial medication on early PD patients. In general, Chinese guideline is similar to the American guidelines except for a few minor aspects. The age divide for DA and LD as initial therapy in China is defined as 65 years while in America it is 70 years old.
Statistical Analysis
We compared the initial therapy and current drug utilization of the 136 PD patients. To estimate the effect of guideline on physicians’ prescription, chi-square test was employed to compare the use of DA and LD as initial therapy. Given delayed effect of the guideline on physicians, January 2007 and January 2010 were defined as two-time dividing points. And patients’ age divide was 65 years old. We compared the two age groups’ use of DA and LD pre-2007, during 2007-2009 and after 2009 as initial treatment. In addition, we also compared the use of DA and LD between class 3-A hospitals and non-class 3-A hospitals by chi-square test. Furthermore, because non-ergot DA was much more expensive than LD, the impact of insurance policy on the use of DA and LD was studied. Non-ergot DA was covered by medical insurance since July 2011. Then we compared the prescription of non-ergot DA before and after 2011 by chi-square test. If the requirements for chi square tests were not met, then Fisher’s exact test was performed. All analyses were performed with SPSS (version 17.0).
Results
This study cohort consisted of 136 patients including 75 males and 61 females. The mean age was 69.68 years. Disease onset ranged from 32 to 84 years old with the mean age of 62.01 years old. Disease duration varied from 1-34 years with the mean length of 7.59 years. The mean value of Hoehn-Yahr stage at PD onset was 1.20. When the study was conducted, Hoehn-Yahr stage ranged from 1-5 with mean value of 2.09. Of all the patients, 22 (16.2%) paid the medical expenses themselves, 84 (61.8%) benefited from medical insurance reimbursement with average costs of RMB 318 per month, the remaining 30 (22.0%) patients enjoyed public health service.
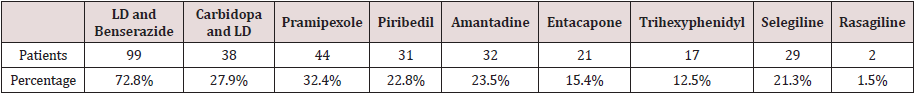
Prevalence of Anti-Parkinsonism Drug (Apd) Use
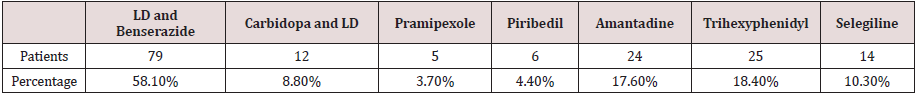
There was a relatively high proportion of APD users received LD and Benserazide. Among the 136 studied patients, 99 (72.8%) patients used LD and Benserazide, 38 (27.9%) received Carbidopa and LD, 44 (32.4%) received pramipexole, 31 (22.8%) received piribedil, 32 (23.5%) took amantadine, 21 (15.4%) took entacapone, 17 (12.5%) took trihexyphenidyl, 29 (21.3%) received selegiline, and 2 (1.5%) received Rasagiline. A total of 116 (85.3%) received at least one LD (LD and Benserazide or Carbidopa and LD) while 55.9% of the studied patients received DA (pramipexole or piribedil). In Table 1, prescriptions for each medication are presented. Prevalence of initial prescription is as follows (Table 2): 58.1% (79) received LD and Benserazide, ranking first among APD; 8.8% (12/136) received Carbidopa and LD, 3.7% (5/136) received pramipexole, 4.4% (6/136) received piribedil, 17.6% (24/136) took amantadine, 18.4% (23/136) received trihexyphenidyl, and 10.3% (14/136) received selegiline. LD was chosen as initial therapy by a total of 91 (66.9%) patients, while only 11 (8.1%) preferred DA as initial prescription.
Effects of Publishing the Guideline on the Prescription Choice
Table 3 summarizes the changes of LD and DA prescriptions before and after the guideline publication. No significant difference between LD and DA prescriptions was found among patients > 65 years old before and after 2007 (P=0.290). In patients < 65 years, initial therapy of LD and DA differed before 2007, during 2007- 2009 and after 2009 (P=0.005). 4.2% patients received DA as initial therapy before 2007, and the proportion significantly increased to 42.9% during 2010-2012 (P=0.028). However, no significance was found between pre-2007 and 2007-2009.
Table 3: DA and LD used as initial prescriptions before and after the publication of the guideline
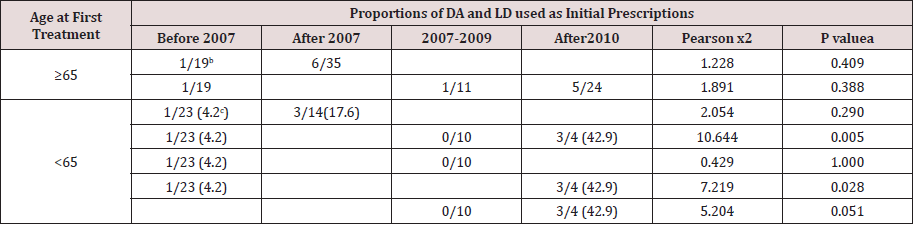
a) Comparison of the number of patients used DA and LD as initial prescriptions at different time-periods using chi-square test or the Fisher’s exact test with one degree of freedom where applicable. Time periods including < 2007 and > 2007 or < 2007, 2007-2009 and >2009.
b) Proportions of DA and LD utilization.
c) Percentage of DA utilization.
The Influence of Hospital Class and Insurance Types on Initial Prescriptions
Initial therapy of LD and DA were compared between Class3-A hospitals and non-Class3-A hospitals. A total of 104 patients were enrolled. 88 patients received initial prescription in Class-3A hospital among whom 76 received LD and 12 used DA. There were 16 patients receiving initial prescription in non-Class 3-A hospitals, among whom 15 received LD and 1 received DA. Initial prescription of DA was not significantly different between Class 3-A hospitals (13.6%) and non-Class 3-A hospitals (6.3%) (P=0.686).
Changes of Non-Ergot Da Prescription Before and After it was Covered by Medical Insurance
It was not until 1/7/2011 that non-ergot DA (pramipexole and piribedil) were formally covered by medical insurance in Beijing. Among the 111 patients in our study before July 2011, 50 (45%) patients received non-ergot DA (28 received pramipexole and 22 received piribedil). From the start of the survey to April 2012, the number of patients receiving non-ergot DA increased to 76 (44 received pramipexole and 32 received piribedil) with a proportion up to 55.9%. However, no significant insurance-related differences were found.
Discussion
Impact of the Publication of the Guideline on Initial Prescription
In our study, the guideline did not significantly influence initial prescription in patients ≥ 65 years old. The outcomes were under expectation since the chances of motor complication induced by LD remained relatively uncommon in old patients. The suggestion of LD prescription in patients ≥ 65 years in the guideline was in line with medical practice before. The guideline dramatically promoted DA utilization in young patients. In general, this statistic results were in agreement with the population-based study in France [7]. However, there existed delay in the implementation of the guideline because no significant differences were found between pre-2007 and 2007-2009. However, initial prescription differed significantly between pre-2007 and 2010-2012. The delay in the practice of guideline could be due to the lack of Chinese physicians’ attention initially. Economy and medical insurance also may have influence on drug prescription. Increased influence of the guideline on Chinese physicians’ practice since 2010 was mainly driven by Movement disorders and Parkinson’s disease study group of Chinese Society of Neurology. Besides, the study group revised and republished the guideline in 2009 which may further enhance the influence of the guideline. It is likely that other factors such as insurance coverage, the publication of related clinical trials and the promotion of pharmaceutical manufactures also contribute to the phenomenon. The guideline improved the proportion of DA utilization in young patients. However, according to our study, a large number of patients < 65 years received LD initially with a proportion of 63.9%, while only 13.7% patients received DA as the initial therapy. This is consistent with surveys abroad [8-11]. It implied that only a minority of physicians prescribed APD based on the guideline and it took time to change clinical practice after the publication of the guideline.
The Influence of Hospital Level and Medical Insurance on Initial Prescription
No significant influence of hospital level and the types of medical insurance on initial prescription were found. Possible reasons were as follows: first of all, daily clinical practice is compliant with the principle of PD medication, both physicians and patients aimed at controlling symptom. Secondly, most patients from class 2-A hospitals we surveyed were from Pinggu traditional Chinese medical hospital where regulatory outpatient service was offered by the physicians from Beijing Hospital of the Ministry of Health. The offered outpatient service can largely improve diagnostic and treatment level. However, some other class 2-A hospitals may offer poor service and patients from those hospitals may not be included in our study. Among the 20 patients who were free of medical insurance, 10 patients were from other provinces. However, these 10 patients had the ability to afford medical costs and had high drug compliance. Finally, another limitation of the presented study should be taken into account since our findings were based on a relatively small number of subjects. Especially, the proportions of initial prescriptions from class 2-A hospitals and patients free of medical insurance were quite small. Two DA (pramipexole and piribedil) started to be covered by medical insurance since 1/7/2011 in Beijing which drove DA utilization modestly (P=0.098). We then performed Fisher exact test with a p value of 0.058. Significant difference may be found when a large number of patients are enrolled. To some extend our study shows that drug compliance and economic burden are needed to be taken into consideration when physicians prescribe medications in China. Moreover, the guideline promotion needs to be supported by medical insurance to some extent.
References
- Katzenschlager R, Lees AJ (2002) Treatment of Parkinson's disease: Levodopa as the first choice. Journal of neurology 249(2): 19-24.
- Thanvi BR, Lo TC (2004) Long term motor complications of levodopa: Clinical features, mechanisms, and management strategies. Postgraduate medical journal 80(946): 452-458.
- Olanow W, Schapira AH, Rascol O (2000) Continuous dopamine-receptor stimulation in early Parkinson's disease. Trends in neurosciences 23(10): 117-126.
- Rajput DR (1993) Accuracy of clinical diagnosis of idiopathic Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry 56(3): 938-939.
- CSTCmdaPsd (2006) The guideline for management of Parkinson’s disease in China. Chin J Neurol 39: 409-412.
- Olanow CW, Watts RL, Koller WC (2001) An algorithm (decision tree) for the management of Parkinson's disease (2001): Treatment guidelines. Neurology 56(5): 1-88.
- Fayard C, Bonaventure A, Benatru I, Roze E, Dumurgier J, et al (2011) Impact of recommendations on the initial therapy of Parkinson's disease: A population-based study in France. Parkinsonism & related disorders 17(7): 543-546.
- Huse DM, Castelli-Haley J, Orsini LS, Lenhart G, Abdalla JA (2006) Patterns of initial pharmacotherapy for Parkinson's disease in the United States. Journal of geriatric psychiatry and neurology 19(2): 91-97.
- Moller JC, Korner Y, Dodel RC, Meindorfner C, Stiasny-Kolster K, et al (2005) Pharmacotherapy of Parkinson's disease in Germany. Journal of neurology 252(8): 926-935.
- Swarztrauber K, Koudelka C, Brodsky MA (2006) Initial pharmacotherapy in a population of veterans with Parkinson disease. Neurology 66(9): 1425-1426.
- Tarrants ML, Denarie MF, Castelli-Haley J, Millard J, Zhang D (2010) Drug therapies for Parkinson's disease: A database analysis of patient compliance and persistence. The American journal of geriatric pharmacotherapy 8(4): 374-383.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...