
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Research Article(ISSN: 2637-6628) 
Anti-aging Activity of Biofield Energy Treated Novel Proprietary Test Formulation by Assessment of Vital Biomarkers in Cerebrospinal Fluid (CSF) in Sprague Dawley Rats Volume 5 - Issue 2
Mahendra Kumar Trivedi1 and Snehasis Jana2*
- 1Trivedi Global, Inc., Henderson, USA
- 2Trivedi Science Research Laboratory Pvt. Ltd, Maharashtra, India
Received: February 11, 2021 Published:February 23, 2021
Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd, Maharashtra, India
DOI: 10.32474/OJNBD.2021.05.000210
Abstract
Over the past few decades, Cerebrospinal Fluid (CSF) biomarkers have been reported as the golden standard for the diagnosis of anti-aging disorders and any neurodegenerative diseases. The study objective was to investigate the effect of Consciousness Energy Healing based novel proprietary test formulation for anti-aging activity using identification of Klotho, tau protein along with serotonin and corticosterone levels. Each ingredient of the test formulation was divided into two parts. One portion was defined as the untreated without any Biofield Energy Treatment, while the other portion was marked as the Biofield Energy Healing Treatment based test formulation, which received the Biofield Energy Healing Treatment by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. All the CSF biomarkers estimation were performed using ELISA assay kit as per manufacturer’s instructions. The level of Klotho was significantly increased by 63% (p≤0.05), 24.5%, 13%, 26.2%, and 41.7% in the experimental test groups viz. G5, G6, G7, G8, and G9, respectively, as compared with the untreated test formulation group (G4). However, the level of corticosterone was found to be decreased by 28.6%, 49.0% (p≤0.05), 18.8%, 51% (p≤0.01), and 62.5% (p≤0.01) in the G5, G6, G7, G8, and G9 groups, respectively, as compared with the G4 group. Similarly, serotonin level was increased by 64.7% and 38% in the Biofield Energy Treated test groups viz. G6 and G8 groups, respectively, as compared with the G4 group. Thus, overall data indicated that the Biofield Energy Healing Treatment would be one of the best approach against aging and its related disorders such as any neuroprotective action, delays aging, improve cognition, age-related diseases, related to aging such as cancer, chronic kidney disease, ataxia, diabetes, and skin atrophy.
Keywords: Biofield Energy Healing; Klotho; Tau protein; Serotonin; Corticosterone; Cognitive decline; Aging
Abbreviations: COPD: Chronic Obstructive Pulmonary Disease; CSF: Cerebrospinal Fluid; MCI: Mild Cognitive Impairment; ROS: Reactive Oxygen Species; ELISA: Enzyme-Linked Immunosorbent Assay; NCCAM: National Center for Complementary and Alternative Medicine; CAM: Complementary and Alternative Medicine
Introduction
Over the age of 65 years, most of the people faced some sort of memory loss, which is supposed to be related with various brain aging biomarkers or some biochemicals. Age-associated memory impairment is considered as normal process, which included various detrimental changes at both molecular and cellular levels [1]. Speed of thinking and attention control are included in normal aging, while abnormal aging included sudden decline in the cognition and is considered as more severe. It includes thinking abilities like rapid forgetting or finding some difficulties in navigating, solving various common sorts of problems, change in conversation expression or behaving outside in the social atmosphere. Abnormal aging is supposed to be very common now-a-days and must have some co- relation with the motor system leading to the excessive tripping, falls or tremor. Mild Cognitive Impairment (MCI) and dementia are the common aging health issues, and the common risk factors for Alzheimer’s disease and related the cognitive decline are type 2 diabetes, high blood pressure, midlife obesity, smoking, depression, little or no mental activity, little or no physical exercise, and many more. According to the WHO, aged population number has been significantly increasing and would be doubled in 2050 [2]. Lifestyle modification is considered as one of the important factor to control the release of biochemicals (corticosterone, tau protein, Klotho protein, serotonin, etc.) which is responsible for aging and memory loss along with generation of free-radicals (Reactive Oxygen Species (ROS) like hydrogen peroxide (H2O2) and superoxide anions (O2-)) [3]. Klotho protein (type I transmembrane protein) is one the pleiotropic protein, which is associated with delaying the aging and enhances cognition. In human, it is encoded as KL gene, controls the organism sensitivity towards insulin and appears to be involved in aging and cognition [4,5]. However, its brain levels decrease with aging [6] naturally that result in decline in the cognitive ability. Thus, it was reported that Klotho would improve the memory and better cognition health, enhances learning with increased aging and can be useful against aging-related cognitive disorder like Alzheimer’s disease. In addition, another important CSF biomarker for aging and cognitive health is the tau protein, also this is one of the best biochemical marker for Alzheimer’s disease [7]. It is the paired helical filament tau protein, which is usually detected and measured in the CSF. Tau protein is a new diagnostic and prognostic in vivo biomarker for Alzheimer’s disease [8]. Corticosteroid and serotonin (5-Hydroxy tryptamine, 5-HT) and all the associated neurotransmitter levels would decrease in the normal aging, which results in neuropsychiatric diseases that may contribute to various behavioral changes, which are generally noticed in the elderly population [9].
On the basis of studies of various important CSF biomarkers responsible for aging and impaired cognitive health, a novel test formulation was designed which was the combination of vital seven ingredients viz. zinc chloride, ferrous sulfate, copper chloride, magnesium gluconate, pyridoxine HCl, Vitamin B12 and Vitamin D3. In additional, the anti-aging action of the test formulation was tested using in vivo important CSF biomarkers such as corticosterone, serotonin level, tau protein and Klotho protein after Biofield Energy Healing Treatment. The test formulation was treated with Biofield Energy Treatment, which was compared with the untreated test formulation in order to study the significance of the Biofield Energy Healing Treatment. Energy Healing therapies are practiced worldwide, and it was accepted as one of the best Complementary and Alternative Medicine (CAM) concept. Biofield Energy Healing Therapies have been reported with wide number of reports for their clinical and non-clinical significance [10,11]. Biofield Energy Healing Therapies have been accepted by the U.S. population and is well defined by National Center for Complementary and Alternative Medicine (NCCAM) as the approach to treat different pathological conditions [12,13]. CAM therapies such as external qigong, Johrei, Reiki, therapeutic touch, yoga, Qi Gong, polarity therapy, Tai Chi, pranic healing, deep breathing, chiropractic/osteopathic manipulation, guided imagery, meditation, massage, homeopathy, hypnotherapy, progressive relaxation, acupressure, acupuncture, special diets, relaxation techniques, Rolfing structural integration, healing touch, movement therapy, pilates, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines in biological systems both in vitro and in vivo. The Trivedi Effect®-Consciousness Energy Healing therapies have been widely accepted worldwide in nonliving materials and living organisms. Consciousness Energy Healing Treatment found to be significant to improve the metal physicochemical properties [14-16], improved crop yield in agriculture science [17,18], microbiology [19-21], biotechnology [22,23], improved bioavailability of many compounds [24- 26], improved skin health [27,28], improved properties of nutraceuticals [29,30], cancer science research [31,32], improved overall bone health [33-35], human health and wellness. With continued advancement of Biofield Energy Healing therapies with unique pharmacological properties, a novel test formulation was designed for its anti-aging action and cognitive health using animal model with respect to the estimation of corticosterone, serotonin, tau protein, and Klotho protein in male Sprague Dawley rats.
DMaterials and Methodse
Chemicals and Reagents
The test formulation is a combination of seven ingredients viz. zinc chloride, ferrous sulfate, copper chloride, magnesium gluconate, pyridoxine HCl, vitamin B12 and vitamin D3. zinc chloride, magnesium (II) gluconate hydrate, pyridoxine hydrochloride, resveratrol, and cyanocobalamin (vitamin B12) were purchased from TCI, Japan. Iron (II) sulphate, copper chloride, and cholecalciferol (vitamin D3) were purchased from Sigma-Aldrich, USA. DetectX® Corticosterone Enzyme Immunoassay kit was used and purchased from ARBOR ASSAYS®, USA. Serotonin Research ELISA was used from Labor Diagnostika Nord (LDN), Germany, while for the estimation of Klotho protein, rat Klotho ELISA Kit purchased from CUSABIO®, USA was used in the experiment. All the other chemicals used in this experiment were analytical grade procured from India.
Laboratory Animals
Randomly breed male Sprague Dawley (SD) rats with body weight ranges between 240 to 400 gm, age with 12-15 weeks were used in this experiment. The animals were purchased from National Institute of Biologicals, Noida, India. Standard normal chow diet (Golden feeds, Mehrauli, New Delhi, India) was provided ad libitum to all groups of animals during experimental phase. Throughout the experiment period, animal room temperature and relative humidity were maintained at 22 ± 2°C and 30% to 70%, respectively. Illumination was controlled to give 12 hours light and 12 hours dark cycle during the 24-hours period. All the animals were acclimatized for the period of 5 days prior to the experiment, and all were accessed once daily for clinical signs, behaviors, morbidity and mortality. All the experimental procedures used in the experiment were in the strict accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The approval of the Institutional Animal Ethics Committee was obtained prior to carrying out the animal experiment.
Experimental Design
After five days of acclimatization, animals were randomized and grouped based on their body weight. A total of nine groups (G) were included i.e. Group 1 (G1) was served as a normal control (i.e. vehicle control), and G2 was served as an aging control; both the groups were received 0.5% Na-CMC, while G3 group animals received resveratrol (200 mg/kg; p.o.) as positive control group animals. G4 group animals received untreated test formulation and G5 group animals received Biofield Energy Treated test formulation at a dose of 130.525 mg/kg. Similarly, G6 group animals received Biofield Energy Treatment (-15 days) per se, G7 animals received Biofield Energy Treated test formulation (-15 days); G8 group defined as Biofield Energy Treated animals + Biofield Energy Treated test formulation (-15 days) and G9 group denoted as Biofield Energy Treatment per se to animals along with the untreated test formulation. The dosing for group G7 and G8 was also initiated on day -15 till end of the experiment. However, G1 to G6 and G9 animals were dosed from day 1 till the end of experiment. All the animals except G1 received D-galactose, daily (500 mg/kg; i.p.) from day 1 to the end of the experiment.
Energy of Consciousness Treatment Strategies
The test formulation was divided into two parts. One part of each ingredient was considered as control without any Biofield Energy Treatment, while another part of each test formulation ingredient received Biofield Energy Healing Treatment (also known as the Trivedi Effect®-Consciousness Energy Healing) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for ~3 minutes. Besides, three groups (G6, G8, and G9) of animals were also received Biofield Energy Treatment under laboratory conditions for ~3 minutes. The unique Biofield Energy transmission was done without touching the samples and animals. Similarly, the control samples were subjected to “sham” healer under the same laboratory conditions for ~3 minutes. The “sham” healer did not have any knowledge about the Biofield Energy Treatment [35]. After that, the Biofield Energy Treated test formulation were kept in the similar sealed condition and used as per the study plan. The Biofield Energy Treated animals were taken back to the experimental room for further proceedings and treatment.
Estimation of Anti-aging Biomarkers using CSF Fluid
Animals (50% of the animals from each group) were fasted overnight on day 66. The remaining 50% animals were dosed with respective formulations and were fasted on day 67. The next day animals were bled, and Cerebrospinal Fluid (CSF) was collected by standard in-house method using stereotaxic instrument for the estimation of Klotho, Tau protein, corticosterone, and serotonin. Klotho, Tau protein, corticosterone, and serotonin were determined with the enzyme-linked immune-sorbent assay kit (USCN, Wuhan, China) with 96-well black polystyrene microtiter plates. ELISA plate was coated overnight at 4°C with the respective monoclonal antibodies, and both antibodies used at concentration specified by manufacturer instructions. After washing with PBS containing 0.05% Tween (PBS-T) 3 times of brain homogenate per well were incubated for about 3 hours at room temperature. After again washing with 3 times with the PBS-T, the horseradish-peroxidase-labeled with specific mAb in the PBS-T was added for 2 hours at room temperature, followed by another 3 washing steps with PBS-T. The optical density was measured with spectrophotometer (USABIO®, USA) at a wavelength of 450 nm. The concentration of Klotho, Tau protein, corticosterone, and serotonin in the test samples was then determined by comparing the optical density of the samples compared with the standard curve.
Statistical Analysis
All the values were represented as Mean ± SEM (standard error of mean). The statistical analysis was performed using SigmaPlot statistical software (v11.0). For two groups comparison Student’s t-test was used. For multiple group’s comparison, one-way analysis of variance (ANOVA) was used followed by post-hoc analysis by Dunnett’s test. Statistically significant values were set at the level of p≤0.05.
Results and Discussion
Estimation of Klotho Protein in CSF
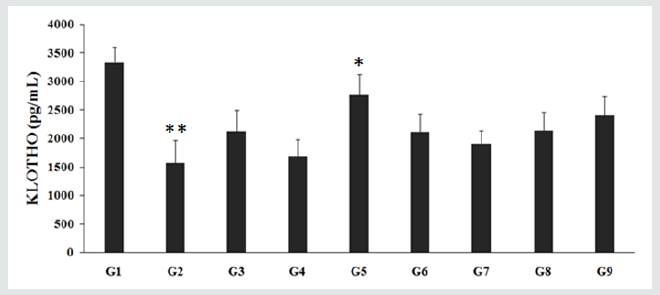
The expression of Klotho protein in the CSF of the normal control (G1) animals was found as 3339.25 ± 273.03 pg/mL, which was significantly (p≤0.01) lower by 52.91% in the aging control group (G2) group i.e., 1572.38 ± 396.83 pg/mL. However, the animals treated with the Biofield Energy per se, reference compound, different combination of the Biofield Energy Treated and untreated test formulation to the Biofield Energy Treated and untreated D-gal induced animals, significantly changed the Klotho level. The positive control group, resveratrol (G3) showed a significant increased Klotho level to 2131.13 ± 375.89 pg/mL. On the other hand, untreated test formulation to the untreated rats (G4) treatment increased Klotho level to 1698.63 ± 283.71 pg/mL. In addition, Biofield Energy Treated test formulation to the untreated rats (G5) treatment showed significantly (p≤0.05) increased Klotho level to 2768.00 ± 354.59 pg/mL (p<0.05). Similarly, the Biofield Energy Treatment to the animals (G6) increased the Klotho level (2115.50 ± 308.19 pg/mL). On the other hand, 15 days pre-treatment of Biofield Energy Treated test formulation (G7) increased the level of Klotho to 1918.63 ± 225.33 pg/mL, while 15 days pre-treatment of Biofield Energy Treated test formulation to the Biofield Energy Treated rats (G8) increased the Klotho level to 2144.25 ± 320.14 pg/mL. The untreated test formulation to the Biofield Energy Treated rats (G9) increased the Klotho level to 2406.75 ± 337.44 pg/mL.
Overall, the level of Klotho in CSF in all the groups was found to be increased after Biofield Energy Treatment. Thus, Klotho level in the test groups viz. G5, G6, G7, G8, and G9 was significantly increased by 63%, 24.5%, 13%, 26.2%, and 41.7%, respectively as compared with the untreated test formulation group. Klotho is a hormone secreted in the human CSF, plasma, and urine, which promotes longevity and influences the onset of several premature senescent phenotypes in mice and humans, including atherosclerosis, cardiovascular disease, stroke and osteoporosis. Klotho benefits might result from modification of certain neurotransmitter receptors, which are present in the brain, known as NMDA. These are involved in the learning and memory. Thus, Biofield Energy Treated test formulation can be significantly used to improve neuroprotective action, delays aging, and improve cognition along with its use against Alzheimer-related toxicity [36]. Thus, the Trivedi Effect® can be defined as the life extension factor against various age-related disorders.
Figure 1: Effect of the Biofield Energy Treated test formulation on Klotho protein. G1- Normal Control; G2- Aging control group (D-Galactose, 500 mg/kg, i.p.); G3-Resveratrol (200 mg/kg); G4-Untreated test formulation; G5- Biofield Energy Treated test formulation; G6- Biofield Energy Treatment per se to the animals (-15 Days); G7- Biofield Energy Treated test formulation (-15 Day); G8- Biofield Energy Treatment per se to animals plus Biofield Energy Treated test formulation (-15 Day); G9- Biofield Energy Treatment per se to animals plus Untreated test formulation. **p≤0.01 vs. G1, *p≤0.05 vs. G4.
Estimation of Tau Protein in Rat CSF after Treatment with the Test Formulation
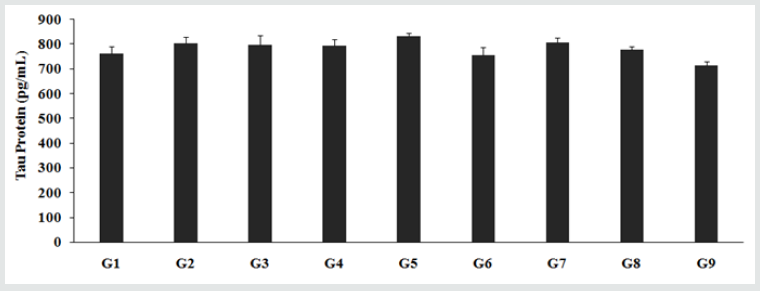
The level of Tau protein in the CSF of rats treated with D-galactose (G2) was 804.62 ± 24.83 pg/mL, which was marginally higher than the control (G1) group 761.44 ± 30.67 pg/mL. However, the animals treated with the Biofield Energy per se, different combination of the Biofield Energy Treated test formulation and untreated test formulation to the Biofield Energy Treated and untreated D-gal induced animals (Group G4 to G9) including reference compound (resveratrol) change the level of Tau protein in the CSF. Tau protein was increased by 5% and 1.6% in the G5 and G7 groups, respectively as compared with the untreated test formulation. However, other test groups showed significant alteration in tau protein as compared with the untreated test formulation. Alzheimer's diseases, one of the common brain disorders are characterized with loss in memory, thinking ability, and behavior. This disease includes plaque formed by beta-amyloidal protein fragments and the tangles produced from tau proteins are very hallmark of brain disorders such as Alzheimer’s disease. The reduced level of tau protein in brain cells results in death of brain and leads to decline its functioning [37]. Thus, the Trivedi Effect® significantly used to improve neuroprotective action, delays aging, and improve cognition against various age-related disorders.
Figure 2: Effect of the Biofield Energy Treated test formulation on Klotho protein. G1- Normal Control; G2- Aging control group (D-Galactose, 500 mg/kg, i.p.); G3-Resveratrol (200 mg/kg); G4-Untreated test formulation; G5- Biofield Energy Treated test formulation; G6- Biofield Energy Treatment per se to the animals (-15 Days); G7- Biofield Energy Treated test formulation (-15 Day); G8- Biofield Energy Treatment per se to animals plus Biofield Energy Treated test formulation (-15 Day); G9- Biofield Energy Treatment per se to animals plus Untreated test formulation. **p≤0.01 vs. G1, *p≤0.05 vs. G4.
Estimation of Corticosterone in Rat CSF after Treatment with the Test Formulation
Figure 3: Effect of the Biofield Energy Treated test formulation on the level of corticosterone in CSF. G1- Normal Control; G2- Aging control group (D-galactose, 500 mg/kg, i.p.); G3-Resveratrol (200 mg/kg); G4-Untreated test formulation; G5-Biofield Energy Treated test formulation; G6- Biofield Energy Treatment per se to the animals (-15 Days); G7- Biofield Energy Treated test formulation (-15 Day); G8- Biofield Energy Treatment per se to animals plus Biofield Energy Treated test formulation (-15 Day); G9- Biofield Energy Treatment per se to animals plus Untreated test formulation. *p≤0.05 vs. G2, **p≤0.01 vs. G4, #p≤0.05 vs. G4.
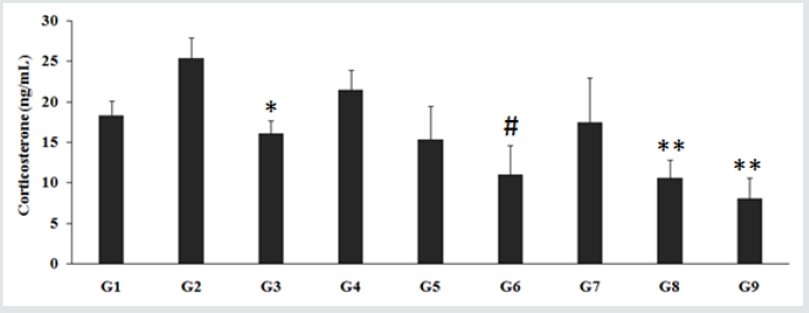
The level of corticosterone in the animal CSF treated with the D-galactose (G2) was 25.37 ± 2.60 ng/mL, which was higher than that of the control (G1) group 18.31 ± 1.75 ng/mL. However, the animals treated with the Biofield Energy per se, different combination of the Biofield Energy Treated test formulation and untreated test formulation to the Biofield Energy Treated and untreated D-galactose induced animals, significant change in the level of corticosterone was reported. The resveratrol treatment (G3) significantly (p≤0.05) decreased the level of corticosterone to 16.03 ± 1.65 ng/mL. The untreated test formulation to the untreated rats (G4) treatment decreased the level of corticosterone to 21.45 ± 2.50 ng/mL. Biofield Energy Treated test formulation to the untreated rats (G5) treatment decreased the level of corticosterone to 15.32 ± 4.10 ng/mL. Similarly, the Biofield Energy Treatment per se to the rats (G6) significantly decreased the level of corticosterone to 10.94 ± 3.68 ng/mL. 15 days pre-treatment of Biofield Energy Treated test formulation (G7) decreased the level of corticosterone, 17.41 ± 5.53 ng/mL. In addition, 15 days pre-treatment of the Biofield Energy Treated test formulation to the Biofield Energy Treated rats (G8) significantly decreased the level of corticosterone to 10.52 ± 2.34 ng/mL. The untreated test formulation to the Biofield Energy Treated rats (G9) significantly decreased the level of corticosterone to 8.04 ± 2.59 ng/mL. The level of corticosterone was significantly reduced by 28.6%, 49.0% (p≤0.05), 18.8%, 51% (p≤ 0.01), and 62.5% (p≤0.01) in G5, G6, G7, G8, and G9 groups respectively, as compared with the untreated test formulation (G4). Thus, the overall results showed that the level was significantly decreased as compared with the untreated test formulation. The data suggested that excess of brain Glucocortioid (GC) secretion would results in brain damage in several areas and lead to loss in cognitive health and increase the aging process. However, corticosterone is the stress hormone [38], and the results of the experiment suggested significance decline after treatment, which showed a significant role in delaying the aging process and improve cognitive health and memory.
Estimation of Serotonin in CSF
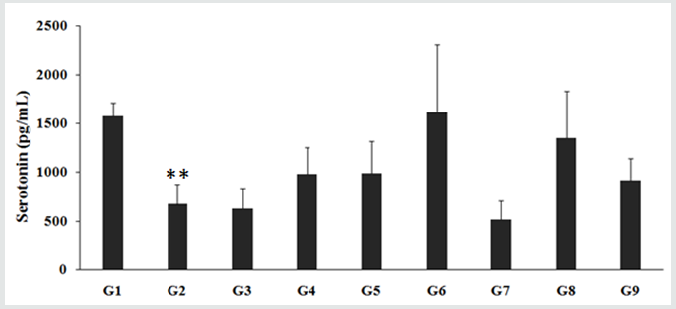
The level of serotonin in the CSF of rats treated with D-galactose (G2) was 673.94 ± 198.02 pg/mL, which was significantly (p<0.01) lower than that of the control (G1) group 1572.41 ± 139.35 pg/mL. However, the animals treated with the Biofield per se, reference compound, different combination of Biofield Energy Treated test formulation and untreated test formulation to the Biofield Energy Treated and untreated D-galactose induced animals changed the level of serotonin in the CSF. Biofield Energy Treatment to the rats (G6) and 15 days pre-treatment of Biofield Energy Treated test formulation to the Biofield Energy Treated animals (G8) considerably increased the level of serotonin to 1610.76 ± 698.73 and 1349.00 ± 484.73 pg/mL, respectively. In comparison with the percentage change, serotonin level was significantly increased by 64.7% and 38% in the G6 and G8 groups, respectively after Biofield Energy Treatment as compared with the untreated test formulation (G4). Serotonin (5-HT) is widely distributed in CNS, and its role is highly important in various neuronal functions like sleep, pain, sexual behavior, feeding, cardiac regulation, and cognition. Loss of serotonin leads to various neuropsychiatric diseases of late life such as depression, Alzheimer's disease, loss of ability of thinking, memory loss, cognitive health, etc., [39]. Thus, the data suggested that Biofield Energy Healing Treatment suggested that the novel test formulation significantly improves the neurodegenerative processes and serotonergic neurotransmission, which would be highly useful against various age-related disorders.
Figure 4: Effect of the Biofield Energy Treated test formulation on level of corticosterone in CSF. G1- Normal Control; G2- Aging control group (D-Galactose, 500 mg/kg, i.p.); G3-Resveratrol (200 mg/kg); G4-Untreated test formulation; G5-Biofield Energy Treated test formulation; G6- Biofield Energy Treatment per se to the animals (-15 Days); G7- Biofield Energy Treated test formulation (-15 Day); G8- Biofield Energy Treatment per se to animals plus Biofield Energy Treated test formulation (-15 Day); G9- Biofield Energy Treatment per se to animals plus Untreated test formulation. **p 0.01 vs. G1.
Conclusion
Based on the obtained results showed that the level of Klotho protein in CSF was significantly increased by 63%, 24.5%, 13%, 26.2%, and 41.7% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the untreated test formulation group (G4). Moreover, the level of corticosterone was significantly decreased by 28.6%, 49.0%, 18.8%, 51%, and 62.5% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G4 group. In addition to, the level of serotonin was increased by 64.7% and 38% in the G6 and G8 groups, respectively after Biofield Energy Treatment as compared with the G4 group. On the basis of animal experimentation of the novel test formulation possesses antiaging properties that could be beneficial for delays aging, improve cognition, age-related diseases, related to aging such as cancer, chronic kidney disease, ataxia, diabetes, and skin atrophy. Therefore, the Biofield Energy Treated test formulation can be used as a Complementary and Alternative Medicine (CAM) can be significantly used to prevent or decline many age-related disorder along with other diseases such as cardiovascular diseases, osteoporosis, dementia, osteoarthritis, hypertension, cancer, Parkinson’s Disease, Chronic Obstructive Pulmonary Disease (COPD), Stress, Asthma, cataract, Age-related Macular Degeneration (AMD), hearing loss, metabolic disorders, and related mobility disability. Additionally, various immune mediated diseases as they all are somehow associated with agerelated pathologies such as Rheumatoid arthritis, Ulcerative colitis, Lupus, Addison Disease, Celiac Disease, Dermatomyositis, Graves’ Disease, Hashimoto Thyroiditis, Multiple Sclerosis, Myasthenia Gravis, Pernicious Anemia, Aplastic Anemia, Sjogren Syndrome, Systemic Lupus Erythematosus, Diabetes, Alopecia Areata, Fibromyalgia, Vitiligo, Psoriasis, Scleroderma, Chronic Fatigue Syndrome and Vasculitis, to improve the overall health and quality of life.
Acknowledgements
The authors are grateful to Dabur Research Foundation, Trivedi Science Research Laboratory Pvt. Ltd., Trivedi Global, Inc., and Trivedi Master Wellness for their support throughout the work.
References
- Raj K, Chanu SI, Sarkar S (2012) Decoding complexity of aging. Cell Dev Biol 1(6): 117.
- World Health Organization (2018) Interesting facts about ageing.
- Fusco D, Colloca G, Monaco MRL, Cesari M (2007) Effects of antioxidant supplementation on the aging process. Clin Interv Aging 2(3): 377-387.
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, et al. (2005) Suppression of aging in mice by the hormone Klotho. Science 309(5742): 1829-1833.
- Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, et al. (2014) Life extension factor klotho enhances cognition. Cell Rep 7(4): 1065-1076.
- Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, et al. (2008) Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia 56(1): 106-117.
- Andreasen N, Vanmechelen E, Van de Voorde A, Davidsson P, Hesse C, et al. (1998) Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer's disease: A community-based follow up study. J Neurol Neurosurg Psychiatry 64(3): 298-305.
- Mecocci P, Cherubini A, Bregnocchi M, Chionne F, Cecchetti R, et al. (1998) Tau protein in cerebrospinal fluid: A new diagnostic and prognostic marker in Alzheimer disease?. Alzheimer Dis Assoc Disord 12(3): 211-214.
- McEntee WJ, Crook TH (1991) Serotonin, memory, and the aging brain. Psychopharmacology (Berl) 103(2): 143-149.
- Movaffaghi Z, Farsi M (2009) Biofield therapies: Biophysical basis and biological regulations. Complement Ther Clin Pract 15(1): 35-37.
- Barnes PM, Powell Griner E, McFann K, Nahin RL (2004) Complementary and alternative medicine use among adults: United States, 2002. Adv Data 343: 1-19.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Fan K Wai (2005) National Center for Complementary and Alternative Medicine Website. J Med Libr Assoc 93: 410-412.
- Trivedi MK, Tallapragada RM (2008) A transcendental to changing metal powder characteristics. Met Powder Rep 63(9): 22-28.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage 4(2): 161.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Effect of biofield energy treatment on physical and structural properties of calcium carbide and praseodymium oxide. International Journal of Materials Science and Applications 4(6): 390-395.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L). Journal of Food and Nutrition Sciences 3(6): 245-250.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of biochemical marker -Glutathione and DNA fingerprinting of biofield energy treated Oryza sativa. American Journal of BioScience 3: 243-248.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3(1): 129.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. J Antivir Antiretrovir 7(3): 83-88.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) An impact of biofield treatment: Antimycobacterial susceptibility potential using BACTEC 460/MGIT-TB System. Mycobact Dis 5(4): 189.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. J Microb Biochem Technol 7(4): 202-205.
- Nayak G, Altekar N (2015) Effect of biofield treatment on plant growth and adaptation. J Environ Health Sci 1(1-2): 1-9.
- Branton A, Jana S (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. International Journal of Clinical and Developmental Anatomy 3(3): 9-15.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. American Journal of Clinical and Experimental Medicine 5(4): 138-144.
- Branton A, Jana S (2017) Effect of the biofield energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3 [25(OH)D3] in rats after a single oral dose of vitamin D3. American Journal of Pharmacology and Phytotherapy 2(1): 11-18.
- Kinney JP, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. American Journal of Life Sciences 5(2): 65-74.
- Singh J, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. American Journal of Pharmacology and Phytotherapy 2(1): 1-10.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) A Systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. International Journal of Bioorganic Chemistry 2(3): 135-145.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) Chromatographic and spectroscopic characterization of the consciousness energy healing treated Withania somnifera (ashwagandha) root extract. European Journal of Biophysics 5(2): 38-47.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4(3): 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253-257.
- Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Influence of biofield treated vitamin D3 on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. International Journal of Biomedical Engineering and Clinical Science 4(1): 6-14.
- Lee AC, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). International Journal of Nutrition and Food Sciences 7(1): 30-38.
- Stutheit ME, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Biofield energy treated vitamin D3: Therapeutic implication on bone health using osteoblasts cells. American Journal of Life Sciences 6(1): 13-21.
- Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, Wang D, et al. (2014) Life extension factor klotho enhances cognition. Cell Rep 7(4): 1065-1076.
- Mohandas E, Rajmohan V, Raghunath B (2009) Neurobiology of Alzheimer’s disease. Indian J Psychiatry 51(1): 55-61.
- De Souza-Talarico JN, Marin MF, Sindi S, Lupien SJ (2011) Effects of stress hormones on the brain and cognition: Evidence from normal to pathological aging. Dement Neuropsychol 5(1): 8-16.
- Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, et al. (1998) Serotonin in aging, late-life depression, and Alzheimer's disease: the emerging role of functional imaging. Neuropsychopharmacology 18(6): 407-430.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




