
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5779
Review Article(ISSN: 2690-5779) 
The Potential of Post-Excavation Novel Ecosystems of Enhancing Vegetation and Rare Plant Species Diversity, Influencing the Ecosystem Services Provision Volume 1 - Issue 4
Gabriela Wozniak*
- Faculty of Natural Sciences Institute of Biology, Biotechnology and Environmental Protection, University of Silesia, Katowice, Poland
Received: March 03, 2021 Published: March 15, 2021
Corresponding author:Gabriela Wozniak, Faculty of Natural Sciences Institute of Biology, Biotechnology and Environmental Protection, University of Silesia, Katowice, Poland
DOI: 10.32474/JOMME.2021.01.000118
Abstract
The pressure of human activity in the industrial centers all over the world has been growing over last centuries. In the urban–
industrial sites the natural environment has been changed significantly. The vegetation cover of the previous ecosystems has
disappeared or has been seriously altered.
Apart from intense environmental transformation some of the sites left after the mineral resources excavation are providing
specific mineral oligotrophic habitats to the urban-industrial environmental ecosystem mosaic. There are some sites which fulfill
the prerequisites of the novel ecosystems. This study conducted on the spontaneous vegetation of the post-excavation novel
ecosystems revealed that the species composition of the spontaneous vegetation is very diverse. The dynamic processes of the
spontaneous vegetation species composition development observed in time and between sites of different area is itself creating
diversity. Vegetation patches are composed from many different plant species including those that are listed as rare or endangered.
The results of analysis conducted for this group of species indicate that the high number habitats this species are derived from, both
in terms of their social-ecological origin and the particular site condition (light, moisture, temperature, acidity), recorded species
are preferring.
The presented high diversity of the recorded spontaneous species composition of vegetation and the diversity vegetation
patches itself is providing the proof how important are the natural processes for enhancing vegetation and rare plant species
diversity. The close relations between biodiversity and ecosystem services provision are already known. This natural process are
particular important in the ecosystem mosaic in the urban–industrial landscape.
Keywords: Vegetation and rare plant species diversity; biodiversity; natural processes; post-excavation novel ecosystems; postcoal mine heaps; urban–industrial landscape
Introduction
The pressure of human industrial activity in the Upper Silesian
Industrial District like in many European industrial centers has been
growing over last centuries. Due to the long industrial development,
the natural environment has been changed significantly. The
exploitation of coal, sand, gravel, limestone and dolomites caused
changes in the surface and landscape [1-5].
Apart from intense environmental transformation some of the
side-products of the mineral resources excavation process, provide
specific mineral oligotrophic habitats to the urban-industrial
environmental mosaic. Such enrichment of the urban-industrial
habitat mosaic has shown that biological systems developing
spontaneously on such sites are assembled of unknown previously
plant and animal species compositions [6-7]. It has been also
revealed that these novel ecosystems are self-sustaining entities,
that provide crucial ecosystem services in the urban–industrial
landscape. The conducted study has shown that spontaneous
flora and vegetation of de novo created habitats like quarries, sand
and gravel pits, heaps and dumps or subsidence reservoirs, are
outstanding due to their floristic richness and the participation of
rare and endangered species [8].
Changes in the natural environment caused by industry
influence on one hand the are connected with the negative social
assessment of the importance of such areas in the landscape,
while on the other hand it has been shown that the restoration of transformed and changed sites is very effective for the benefit
of nature and economy when the natural processes, i.e. natural
succession are involved [9-15]. For post-industrial, post-excavation
sites, for which the ecological threshold has been crossed the
effective restoration in its classical understanding is not possible
any more[16-17]. Such sites are called novel ecosystems. The novel
ecosystems are developing on unusual (often on de novo created)
habitats. On such habitats, the vegetation and further on ecosystems
are composed from plant and animal species assemblages linked
by relations which have not been unknown previously from natural
and semi-natural ecosystems [16-17].
The aim of this study was to present some the ecological
characteristics and diversity of the spontaneous vegetation and
of the recorded rare endangered and protected species which
have spontaneously colonize, overgrow the vegetation of post coal
mine and post-excavation habitats and discuss their importance
for maintaining biodiversity in the aspect of ecosystem services
provision in densely populated urban-industrial landscapes
(environmental, habitat and ecosystem) mosaic.
The Study Site Area
Floristic and vegetation records have been conducted during the main stream study focused on the analysis of the conditions influencing of the diversity of spontaneous vegetation on post coalmine heaps in Upper Silesia south Poland [18]. The Silesian Upland is a geographical region located in southern Poland and the largest industrial center in Poland. In south Poland the main branches of industry are the coal, sand, gravel, and limestone mining. The longtime of excavation has changed the landscape and the natural environment conditions including hydrology and water coursers on the surface [4, 19-22].
Results
The vegetation diversity and ecological spectrum of rare plant species
Post-industrial particular post-mineral exploitation sites,
especially those subjected to spontaneous succession are
characterized by specific habitat conditions, and thus unique
biocoenoses arise on those habitats [18]. They are often
characterized by a high number of species (biodiversity). Over
500 plant species were recorded on the post-coal mine heaps and
sedimentation [18, 23-25]. Apart from the post-coal mine sites
the vascular plant species flora of quarries, gravel and sand pits,
post coal-mine heaps and dumps present high floristic richness,
including the occurrence of rare and endangered species.
The long term study on the conditions influencing of the
diversity of spontaneous vegetation on post coal-mine heaps in
Upper Silesia have shown that vegetation diversity is changing
in time. The spontaneous vegetation is most diverse in the initial
stages of post-coal mine heaps ecosystem development (Figure 1).
Figure 1: The changes in the spontaneous vegetation diversity on post-coal mine heaps of different age. Explanations – socio-ecological groups: forest species (Cl. Vaccinio-Piceetea, Querco-Fagetea, Alneteaglutinosae); meadow species (Cl. Molinio-Arrhenatheretea); grassland species (Cl. Festuco-Brometea, Sedo-Scleranthetea, Nardo-Callunetea); karst species (Cl. Asplenietea, Violeteacalaminariae,Thlaspietea); fringe species (Cl. Epilobieteaangustifolii, Trifolio-Geranietea); ruderal species (Cl. Artemisietea, Agropyretea, Plantaginetea, Agrostieteastoloniferae); salt marsh species (Cl. Zosteretea,Ruppietea, Astereteatripolii, Honckenyo-Elymetea, Cakiletea,Ammophiletea); agriculture weeds (Cl. Chenopodietea, Secalietea); peatbog species (Cl. Montio- Cardaminetea, Scheuchzerio-Caricetea, Oxycocco-Sphagnetea); rush and aquatic species (Cl. Phragmitetea, Isoëto-Nanojuncetea, Bidenteteatripartitae, Lemnetea,Utricularietea, Potamogetonetea).
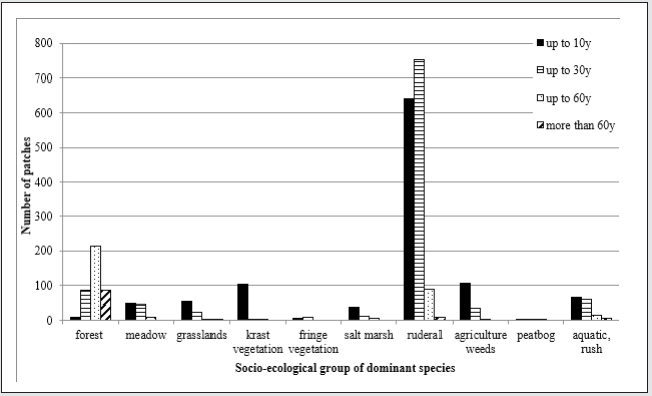
The study conducted on heaps of different area has shown that the vegetation is the most diverse on the youngest heaps. On the coal mine heaps of the age up to 10 years the most commonly occurring patches have represented socio-ecological groups of the ruderal, segetal agricultural weeds and karst vegetation types. The coal mine heaps of the age up to 30 years were covered most often by patches that have represented the ruderal, wetland and woodland forest and aquatic vegetation (Figure 1).
Figure 2: The changes in the spontaneous vegetation diversity on post-coal mine heaps of different area. Explanations – socio-ecological groups: forest species (Cl. Vaccinio-Piceetea, Querco-Fagetea, Alneteaglutinosae); meadow species (Cl. Molinio-Arrhenatheretea); grassland species (Cl. Festuco-Brometea, Sedo-Scleranthetea, Nardo-Callunetea); karst species (Cl. Asplenietea, Violeteacalaminariae, Thlaspietea); fringe species (Cl. Epilobieteaangustifolii, Trifolio- Geranietea); ruderal species (Cl. Artemisietea, Agropyretea, Plantaginetea, Agrostieteastoloniferae); salt marsh species (Cl. Zosteretea, Ruppietea, Astereteatripolii, Honckenyo-Elymetea, Cakiletea, Ammophiletea); agriculture weeds (Cl. Chenopodietea, Secalietea); peatbog species (Cl. Montio-Cardaminetea, Scheuchzerio-Caricetea, Oxycocco-Sphagnetea); rush and aquatic species (Cl. Phragmitetea, Isoëto-Nanojuncetea, Bidenteteatripartitae, Lemnetea, Utricularietea, Potamogetonetea).
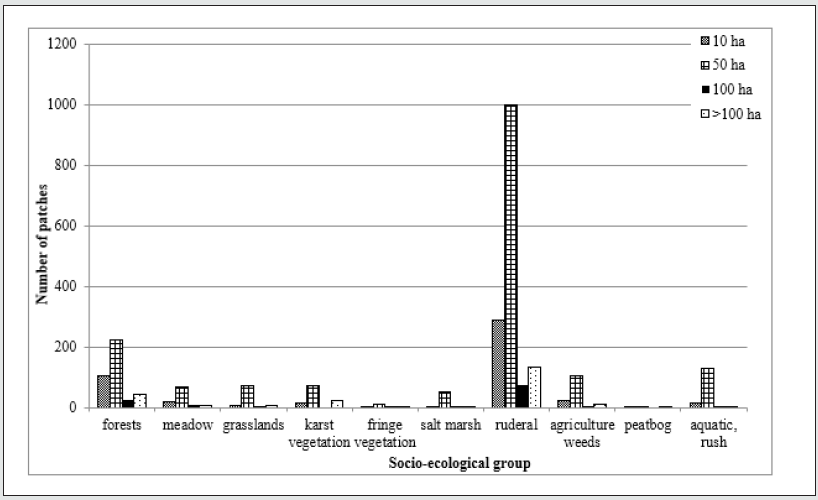
Comparing the vegetation diversity on coal mine heaps of
different area has shown that the heaps of the area up to 50 ha are
the most diverse. The vegetation patches represented all ten main
vegetation types (Figure 2). The most frequent were the patches
of ruderal, woodland forest and aquatic, woodland vegetation.
The patches of other vegetation types have been present but not
the most frequent. All the analyzed vegetation types have been
recorded on the smallest up the 10 ha studied heaps. However, the
diversity has been mostly influenced by the presence of the salt
marsh, fringe, peat bog, moores and grassland vegetation patches
(Figure 2).
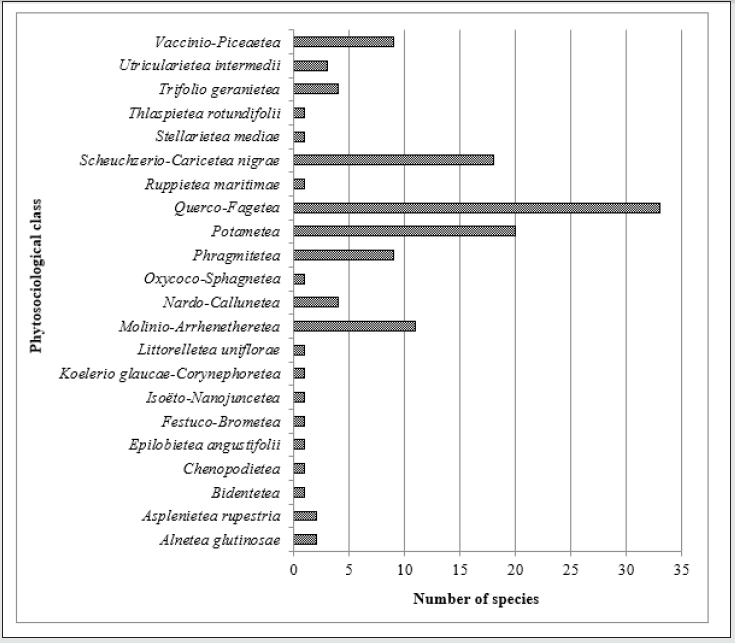
Recorded rare plant species occurring in the vegetation on
the studied post-excavation sites have been divided into groups
depending on the vegetation type they represent according to
Oberdorfer et al. [26]. The most numerous recorded rare plant
species represent deciduous beach forest of the Querco-Fagetea
class. The second abundant group of recorded rare plant species
represent the water (Potametea) and peat bog (Scheuchzerio-
Caricetaeanigrae) class of vegetation type (Figure 3).
Among listed species there are some which were recorded on
different types of objects. They were: Centaurium erythraea subsp.
erythraea, Epipactisatrorubens, E. palustris, Eriophorumlatifolium,
Malaxismonophyllos, Melampyrumsylvaticum, Myricaria germanica,
Najas marina, Neottia nidus-avis, Polypodium vulgare, Pyrola
chlorantha, P. minor, Schoenoplectustabernaemontani and
Utricularia australis.
Figure 3: The number of rare plant species recorded in the vegetation patches on the studied post-excavation sites representing vegetation type groups depending on their classification in the natural and semi-natural ecosystems according to Oberdorfer et al. [26].
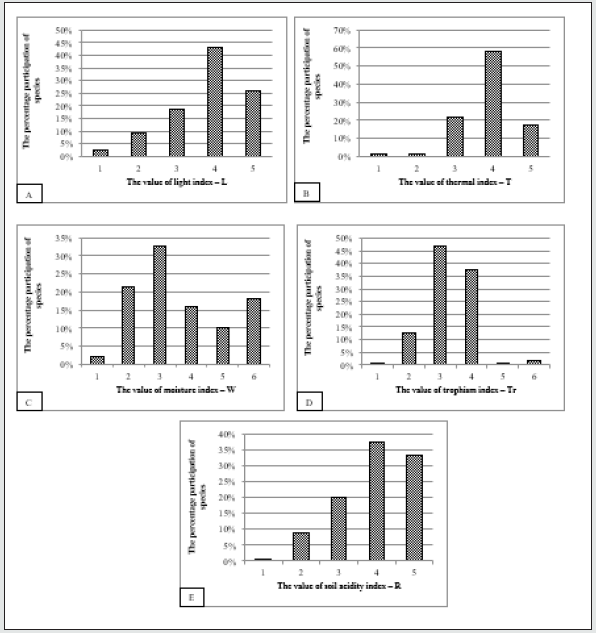
The analysis of abiotic habitat conditions where the recorded
rare plant species occurring in the vegetation on the studied postexcavation
sites, are coming from has been divided into groups
depending on the preferred circumstances in terms of light,
moistures, temperature and reaction parameters (Figure 4).
Most of rare plant species recorded in the vegetation patches on
the studied post-excavation sites are known to occupy the places of
moderate and full light conditions. Less of the analyzed plant species
occupy the places of half shade and moderate shade light availability
(Figure 4). In terms of temperature index requirements the most of
the rare plant species are known to grow in moderately cold climatic
conditions, lower regions, northern lowlands and special microhabitats
- raised bogs and moderately warm climatic conditions,
mainly lowlands and lowlands. Less species has been recorded
representing the warmest regions and micro-habitats, thermally
advantaged areas and the coldest areas of the country, mainly
the alpine and subalpine floors (Figure 4). Analyzing the habitat
moisture value of the rare plant species recorded in the vegetation
patches on the studied post-excavation sites the numerous group
of species is representing habitats moderately moist and water
conditions. The following groups represent dry habitats and very
moist habitats. The differentiation of the trophism index among
the analyzed species has shown that the highest number for plants
are representing the moderately rich (mesophrophic) soils (water)
- mixed forest, tall, acidophilous oak and beech forests and rich
(eutrophic) soils (water) - lowland, fertile beech forests as well as
poor (oligotrophic) soils (water) - fresh pine coniferous forests.
Much less species represents the very rich (extremely fertile, overfertilised)
soils (water), (Figure 4). In terms of soil acidity index,
the presence of species occurring on alkaline soils is justified by the
presence of the largest number of the recorded species, followed
by those that are known from the sub-neutral to neutral, 6≤pH<7
habitats. The least group of the recorded species represent the
highly acidic soils, pH<4 site conditions.
Figure 4: The participation of recorded rare species in relations to the value of selected ecological indicators: A – light
index (L), B – thermal index (T), C – moisture index (W), D – trophism index (Tr), E – soil acidity index (R).
Explanations: Light index (L): 1 – deep shade, 2 – moderate shade, 3 – half shade, 4 – moderate light, 5 – full light.
Thermal index (T): 1 – the coldest areas of the country, mainly the alpine and subalpine floors, 2 – moderately cold
areas, mainly the subalpine and upper regions, 3 – moderately cold climatic conditions, lower regions, northern
lowlands and special micro-habitats - raised bogs, 4 – moderately warm climatic conditions, mainly lowlands and
lowlands, 5 – the warmest regions and micro-habitats, thermally advantaged areas. Moisture index (W): 1 – habitats
very dry, 2 – habitats dry, 3 – habitats moderately moist, 4 – habitats very moist, 5 – habitats wet, 6 – water. Trophism
index (Tr): 1 – extremely poor (extremely oligotrophic) soils (water) – raised bogs, loose sands, dry coniferous forests,
2 – poor (oligotrophic) soils (water) – fresh pine coniferous forests, 3 – moderately rich (mesophrophic) soils (water)
– mixed forest, tall, acidophilous oak and beech forests, 4 – rich (eutrophic) soils (water) – lowland, fertile beech
forests, 5 – very rich (extremely fertile,) soils (water), 6 – over-fertilised soils, water. Soil acidity index (R): 1 – highly
acidic soils (pH<4), 2 – acidic soils (4≤pH<5), 3 – moderately acidic (5≤pH<6), 4 – subneutral to neutral (6≤pH<7), 5
– alkaline (pH>7).
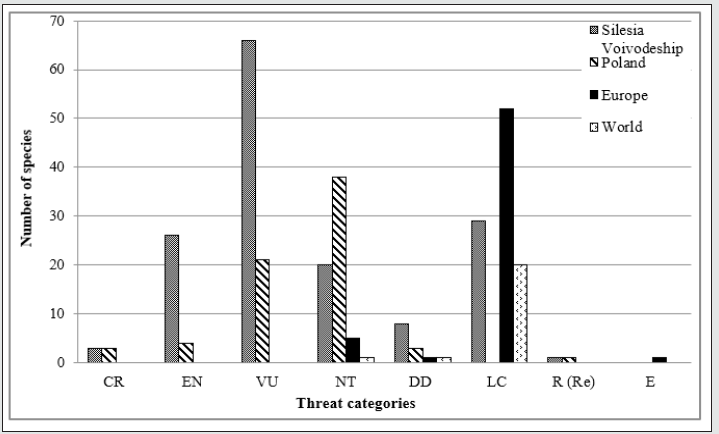
The analysis of rare plant species recorded in the vegetation
patches on the studied post-excavation sites presenting the
participation of species with different threat categories. The largest
group of species is vulnerable (V) and near threatened (NT) both in
the regional and country scale. In the Europe and world scale most
of recorded species are least concern (LC), (Figure 5).
Some of those objects have become included into the UNESCO
World Heritage List [27]. The open cast sand pits in south Poland
appear to be habitat that enables the development of numerous
population of one of the representative of the orchid family – Liparis
loeselii[28]. This species is included in Annex II of the Habitats
Directive [29].
Figure 5: The participation of rare plant species recorded in the vegetation patches on the studied post-excavation sites in respect to different threat categories. Explanations: Threat categories:CR – critically endangered, EN – endangered, VU – vulnerable, NT – near threatened, DD – data deficient, LC – least concern, R (Re) – regionally extinct, E – extinct.
Discussion
Ecosystem services, vascular plant species and vegetation diversity
The relationship between habitat variability, species diversity,
biodiversity, ecosystem functioning and ecosystem services is still
discussed and intensely studied [30]. It is very often difficult to
estimate what is the role of individual species in providing specific
ecosystem services [31]. The role of the key note species has been
proven to influence ecosystem services the up to now. Scientists
undertake many projects regarding this issue. Species diversity
plays vitally important role in increasing resistance to invasive
species [32]. The long-term studies show that high species and /
or functional diversity provides higher stability to the ecosystems,
resulting in greater stability and ability to provide wider range of
ecosystem services [33-34].
Vegetation and species diversity apart from physical, mental,
spiritual and aesthetic benefits for health is influencing also the
atmosphere regulation, pests or pollinators abundance, prevention
of erosion and stimulation of soil-forming processes [30]. It has
been proven that the increase and protection of biodiversity
particular rare and endangered plant species and vegetation, causes
an increase in other ecosystem services [3, 14, 35-37]. In recent
years, there are more and more papers regarding the valuation
of ecosystem services both in areas used by human and protected
areas, e.g. [38]. The assessment of the costs necessary to preserve
ecosystem services is met by Landscape Equivalency Analysis –
ARK, which makes it possible to balance economic activities with
ecosystem protection goals.
According to Bacler-Żbikowska and Nowak [39] the vascular
plant species recorded in post-industrial sites of the Silesian
Upland covers 7,4 % of the number of species listed in the flora
of the Silesian Province. The plant species observed on postindustrial
sites of the Silesian Upland are varied and represent
different functional groups. Particularly valuable is the presence
of species that represent the Orchidaceae family. The list of orchid
species growing on Silesian post-industrial sites cover about 50%
of all orchid species occurring in Poland. All the representatives
of the Orchidaceae family are protected in Poland. Additionally
the presence of endangered and rare species in the Silesian postindustrial
sites provide the proof that the habitat conditions of
post-industrial areas are suitable as secondary habitats for these
species. The long term study suggests that the post-industrial
areas support the spontaneous colonization, establishment and
survival of rare, endangered and protected plant species as results
these sites have become refugee for species which have been out
competed and disappear from natural habitats. In the Olkusz
Ore District more than 800 species of vascular plants have been
recorded, what represent about 20% of Poland’s flora [8]. In some
European countries, specific forms of nature protection like SSSI
are introduced for particularly valuable wild life post-industrial
areas.
Many ecosystem functions of the biological systems that develop on post-industrial sites could provide ecosystem services in densely populated urban and industrialized lands [15]. In the urban-industrial space, the spontaneous development and constant evolution of new ecological systems in some of the post-industrial areas, directly contribute to biodiversity increase and in this way to the improvement of the ecosystem mosaic functioning and though the quality of human life condition. The recoded organisms’ diversity results from the verity of specific micro-habitats of the post-industrial sites. In addition to plants, the primary producers, which are most often analyzed, both in Poland and other European countries, the biocenotic function for various animal groups is emphasized [40-43]. Due to the intense colonization and evolution process which take place spontaneously on the neglected postindustrial sites, a specific “network of life” is established increasing the cover of green areas. This network has also encouraged the flow of genes in areas which are known of significant habitats fragmentation.
Conclusion
The presence of high diversity of the recorded spontaneous
species composition of vegetation and the diversity of vegetation
patches itself growing on the post-coal mine and post-excavation
has to be considered as a factor during environmental management
projects, when the novel ecosystems habitats are considered. The
presented study is providing the proof of how important are the
natural processes for enhancing vegetation and rare plant species
diversity. The vegetation species composition is governing the
character of the following below and above ground ecosystem
processes reflecting the close relations between biodiversity
and ecosystem functioning and ecosystem services provision.
This natural process are particular important in the ecosystem
mosaic in the urban–industrial landscape. The natural processes
are maintaining the ecosystem and environmental function lost
because of human activity. The presented results show that only
time must be given to get the ecological processes back.
The ecological features of the analyzed representatives
present a wide spectrum, which is confirmed by the diversity of
habitat conditions characterizing post-industrial areas. The postindustrial
areas novel ecosystems habitat conditions help the rare
and endangered plant species to survive in the urban–industrial
landscape.
References
- Dulias R, Hibszer A (2004) Województwo ślą Przyroda. Gospodarka. Dziedzictwo kulturowe. Wydawnictwo Kubajak. Kraków, Poland.
- MEA – Millennium Ecosystem Assessment (2005) Ecosystems and Human Well-Being: Synthesis. Island Press. Washington DC, USA.
- de Groot RS, Alkemade R, Braat L, Hein L, Willemen L (2010) Challenges in integrating the concept of ecosystem services and values in landscape planning, management and decision making. Ecological Complexity 7(3): 260-272.
- Dulias R (2018) Geografia fizyczna Wyżyny Ślą Podręczniki i Skrypty Uniwersytetu Śląskiego w Katowicach nr 201. WydawnictwoUniwersytetuŚląskiego. Katowice. pp. 1-216.
- Kompała-Bąba A, Sierka E, Dyderski MK, Bierza W, Magurno F, et.al.(2020) Do the dominant plant species impact the substrate and vegetation composition of post-coal mining spoil heaps? Ecological Engineering 143: 105685.
- Błońska A, Kompała-Bąba A, Sierka E, Besenyei L, Magurno F, et.al. (2019) Impact of selected plant species on enzymatic activity of soil substratum on post-mining heaps. Journal of Ecological Engineering 20(1): 138-144.
- Błońska A, Kompała-Bąba A, Sierka E, Bierza W, Magurno F, et.al.(2019) Diversity of vegetation dominated by selected grass species on coal-mine spoil heaps in terms of reclamation of post-industrial areas. Journal of Ecological Engineering 20(2): 209-217.
- Nowak T, Jędrzejczyk-Korycińska M, Kapusta P, Szarek-Łukaszewska G (2015) Characteristics in the vascular plant flora in the Olkusz Ore-bearing Region. In: Godzik B (ed) Natural and historical values of the Olkusz Ore-bearing Region. W. Szafer Institute of Botany. Polish Academy of Sciences. Kraków, poland pp. 147-166.
- Woźniak G, Kompała A (2000) Gatunki chronione i rzadkie na nieużytkach poprzemysł In: Nakonieczny M (ed.) Problemy środowiska i jego ochrony. Wydawnictwo Uniwersytetu Śląskiego 8: 101-109.
- Woźniak G, Kompała A (2000) Rola procesów naturalnych w rekultywacji nieużytków poprzemysł InżynieriaEkologiczna 1: 87-93.
- Bruggeman DJ, Jones ML, Lupi F, Scribner KT (2005) Landscape Equivalency Analysis: Methodology for Estimating Spatially Explicit Biodiversity Credits. Environmental Management 36(4): 518-534.
- Kremen C (2005) Managing ecosystem services: what do we need to know about their ecology? Ecology Letters 8(5): 468-479.
- Woźniak G, Cohn EVJ (2007) Monitoring of spontaneous vegetation dynamics on post coal mining waste sites in Upper Silesia, Poland. In: Sarsby R, Felton A (eds) Geotechnical and Environmental Aspects of Waste Disposal Sites. Taylor and Francis Group, London pp. 289-294.
- Larondelle N, Haase D (2012) Valuing post-mining landscapes using an ecosystem services approach – An example from Germany. Ecological Indicators 18: 567-574.
- Woźniak G, Sierka E, Wheeler A (2018) Urban and Industrial Habitats: How Important They Are for Ecosystem Services. In: Hufnagel L (ed.) Ecosystem Services and Global Ecology pp. 169-194.
- Hobbs RJ, Higgs E, Harris JA (2009) Novel ecosystems: implications for conservation and restoration. Trends in Ecology & Evolution 24(11): 599-605.
- Hobbs RJ, Higgs ES, Hall CM (2013) Defining novel ecosystems. In: Hobbs RJ, Higgs ES, Hall CM (eds) Novel Ecosystems: Intervening in the New Ecological World Order. John Wiley & Sons, Ltd, USA.
- Woźniak G (2010) Zróżnicowanie roślinności na zwałach pogórniczych Górnego Ślą InstytutBotanikiim W Szafera PAN. Kraków, poland pp. 1-320.
- Singh RS (2000) Revegetation of coal mine overburden dump (OBD) slopes by aromatic grasses. Journal of Medicinal and Aromatic Plant Sciences 22(1B): 506-509.
- Pieczyrak J (2010) Characteristics and engineering usage of waste from coal mining. Architecture Civil Engineering Environment 3(1): 77-84.
- Poros M, Sobczyk W (2013) Rewitalizacja terenu pogórniczego po kopalni surowców skalnych na przykładzie kamieniołomu Wietrznia w Kielcach. Rocznik Ochrona Środowiska 15(3): 2369-2380.
- Poros M, Sobczyk W (2014) Kierunki rekultywacji terenów pogórniczych obszaru chęcińsko-kieleckiego w kontekście ich wykorzystania w aktywnej edukacji geologicznej. RocznikOchronaŚrodowiska 16(1): 386-403.
- Woźniak G (1998) Primary succession on the sedimentation pools of coal mine. In: Faliński JB, Adamowski W, Jackowiak B (eds) Synanthropization of plant cover in new Polish research. Phytocoenosis Vol. 10 (N.S.) Supplementum Cartographiae Geo botanicae 9: 189-198.
- Woźniak G (2006) Colonisation process on coal mine sedimentation pools (Upper Silesia, Poland). Polish Botanical Studies, Poland 22: 561-568.
- Rostański A (2006) Spontaniczne kształtowanie się pokrywy roślinnej na zwałowiskach po górnictwie węgla kamiennego na Górnym Ślą Prace Naukowe Uniwersytetu Śląskiego w Katowicach. Nr 2410. Wydawnictwo Uniwersytetu Śląskiego. Katowice, poland.
- Oberdorfer E, Müller T, Korneck D, Lippert W, Markgraf-Dannenberg I et.al. (1990) Pflanzensoziologische Excursionsflora. 6 Auflage. Ulmer, Stuttgart, Germany.
- https://smzt.pl/2018/03/28/obiekt-unesco-w-trojwymiarze/
- Błońska A (2010) Siedliska antropogeniczne na Wyżynie Śląskiej jako miejsca występowania rzadkich i zagrożonych gatunków torfowiskowych klasy Scheuchzerio-Caricetea nigrae (Nordh. 1937) R. Tx. 1937. Woda-Środowisko-Obszary Wiejskie 10(1): 7-19.
- Habitats Directive (2019).
- Balvanera P, Quijas S, Martín-López B, Barrios E, Dee L (2016) The links between biodiversity and ecosystem services. In: Potschin M, Haines-Young R, Fish R, Turner K (eds) Routledge Handbook of Ecosystem Services. Taylor & Francis Group p. 45-59.
- de Groot R, Jax K, Harrison P (2016) Links between Biodiversity and Ecosystem Services. In: Potschin M, Jax K (eds) OpenNESS Ecosystem Services Reference Book. EC FP7 Grant Agreement no. 308428.
- Wilsey BJ, Polley HW (2002) Reductions in grassland species evenness increase dicot seedling invasion and spittle bug infestation. Ecology Letters 5(5): 676-684.
- Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proceedings of the National Academy of Sciences of the United States of America 96(4): 1463-1468.
- Chmura D, Molenda T (2007) Nowe stanowisko omiegu górskiego Doronicum austriacum na Górnym Śląsku. Chrońmy przyrodę ojczystą 63(1): 20-24.
- Hockings M (2003) Systems for assessing the effectiveness of management in protected areas. BioScience 53(9): 823-832.
- Hockings M, Stolton S, Leverington F, Dudley N, Courrau J (2006) Evaluating Effectiveness: A framework for assessing management effectiveness of protected areas. 2nd IUCN. Gland, Switzerland and Cambridge, UK.
- Zipper CE, Burger JA, Skousen JG, Angel PN, Barton CD(2011) Restoring Forests and Associated Ecosystem Services on Appalachian Coal Surface Mines. Environmental Management 47: 751-765.
- Wieliczko B (2016) Wykorzystanieusług ekosystemów w zarządzaniu zasobami naturalnymi w rolnictwie. Studia i Prace Wydziału Nauk Ekonomicznych i Zarządzania Uniwersytetu Śląskiego 46(2): 135-144.
- Bacler-Żbikowska B, Nowak T (2021) The role of post-industrial sites in maintaining species diversity of rare, endangered and protected vascular plant species on the example of the Silesian Upland. In: Woźniak G, Dyczko A, Jagodziński AM (eds.) Mining industry responding to environmental challenges of the Anthropocene: Green Scenarios. Taylor & Francis Group, USA.
- Tropek R, Konvicka M (2008) Can quarries supplement rare xeric habitats in a piedmont region? Spiders of the Blansky les Mts Czech Republic. Land Degradation and Development 19(1): 104-114.
- Tropek R, Kadlec T, Karesova P, Spitzer L, Kocarek P, et.al.(2010) Spontaneous succession in limestone quarries as an effective restoration tool for endangered arthropods and plants. Journal of Applied Ecology 47(1): 139-147.
- Tropek R, Kadlec T, Hejda M, Kocarek P, Skuhrovec J, Malenovsky I, et.al.(2012) Technical reclamations are wasting the conservation potential of post-mining sites. A case study of black coal spoil dumps. Ecological Engineering 43: 13-18.
- Tropek R, Cerna I, Straka J, Cizek O, Konvicka M (2013) Is coal combustion the last chance for vanishing insects of inland drift sand dunes in Europe? Biological Conservation 162: 60-64.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




