Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5779
Research Artical(ISSN: 2690-5779) 
Syngas Compositions, Cold Gas and Carbon Conversion Efficiencies for Different Coal Gasification Processes and all Coal Ranks Volume 1 - Issue 2
Said MA Ibrahim* and Mostafa EM Samy
- Faculty of Engineering, Department of Mechanical Engineering, Al-Azhar University, Egypt
Received: March 13, 2020 Published: June 11, 2020
Corresponding author: Said MA Ibrahim and also in citation, Faculty of Engineering, Department of Mechanical Engineering, Al- Azhar University, Egypt
DOI: 10.32474/JOMME.2020.01.000109
Abstract
This Paper presents comparison of syngas compositions, for all coal ranks, as produced from different types of coal gasification processes currently in use, namely entrained flow, fluidized bed, and fixed bed gasifiers. Cold gas and carbon conversion efficiencies were investigated. The syngas composition varies with the applied gasification process. The importance of this research arises from the fact that gasifiers produce the environmentally clean fuel required to run any Integrated Gasification Combined Cycle power system. A procedure was conducted to get estimates for bituminous coal. It is important to have knowledge of the chemical reactions which take place in each gasifier and the raw syngas produced from the specific reaction involved to develop an Integrated Coal Gasification Combined Power Generation plant with CO2 recovery in order to increase the cycle efficiency and mitigate CO2 emission and other pollutants. The results indicate that the entrained flow gasifier is the dominant one.
Keywords: Syngas; Coal gasification; Cold gas efficiency; Coal conversion efficiency; Gasifiers
Introduction
Gasifiers in an Integrated Gasification Combined Cycle power plant (IGCC) are the heart of the system since they are the producers of the syngas fuel required to operate the plant. Their efficiency and availability are determining factors not only for their design but also for the IGCC system. The study and comparison of different gasifiers are essential for the efficient operation of IGCC plants.
In gasification, organic (carbonaceous) feeds are converted into CO, CO2, and H2 by processing the feed at elevated temperatures (>7000C), without combustion, with a controlled amount of oxygen and/or steam. The produced gas mixture (synthesis gas or syngas) is a fuel. The power generated from the combustion of the syngas, is a source of renewable energy if the obtained gaseous products are produced from a source other than a fossil fuel, e.g. biomass [1].
The main advantage of coal gasification is the utilization of the syngas which is actually more efficient in its burning as compared to the direct combustion of the solid coal because of: (1) the possibility of combustion at higher temperatures, i.e. increasing the thermal efficiency, (2) its use in fuel cells, (3) the possibility to produce methanol and hydrogen, and (4) its conversion via the Fischer–Tropsch (FT) process into a range of synthesis liquid fuels suitable for use in gasoline or diesel engines [1]. In addition, coal is still a virgin resource because of its large reserves in many countries around the world and thus it is greatly more secured than oil and natural gas. It is not subjected to the unexpected price variations in comparison.
The high temperature process produces corrosive ash elements, including metal chlorides and potassium salts, which allow clean gas production from otherwise problematical fuels [1]. However, the ash content in coal is the top important factor in selecting coal since it represents the greatest problem in the operation and performance of a slagging gasifier. Gasifiers need to be efficient for the purpose of increasing the availability of the IGCC system, which is a demanding requirement. It is essential to avoid high ash content in the chosen coal because its melting needs more heat, and hence additional coal and CO are combusted. This increases the amount of CO2 in the syngas and a reduction in the cold gas efficiency as a consequence of the high ash content.
Coal gasification power plants are cleaner compared to pulverized coal (PC) combustion plants, since they produce less sulphur and nitrogen oxides pollutants. Therefore, gasification is an appealing technology which enables the utilization of both relatively inexpensive and expensive coal reserves, in addition to reducing down the environmental impact. In fact, the increased mounting interest in coal gasification announces two changes in the electricity generation arena: (1) the maturity of coal gasification technology, and (2) the incredibly low air emissions from IGCC power stations, and better lower cost control of greenhouse gases than that for other coal fired plants [1].
The Gasification Process
The real reactions associated with the gasification process
are immensely complicated and change with the feed material
properties [2].
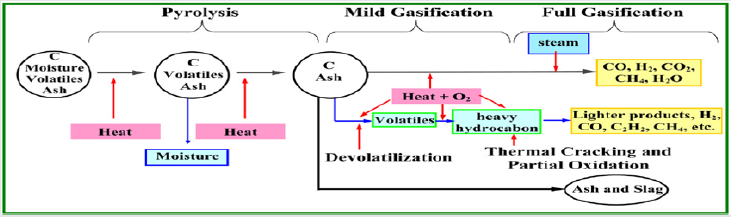
The gasification of coal comprises three chief steps, as shown
in Figure 1: (a) pyrolysis and devolatilization, (b) volatiles cracking
and combustion, and (c) char gasification. These processes are
explained briefly.
Pyrolysis and Devolatilization
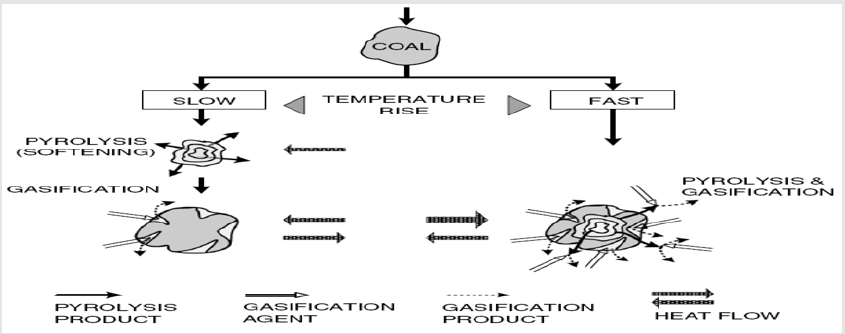
The interaction between pyrolysis and gasification under various conditions of heating is depicted in Figure 2. If the heating is slow then the pyrolysis reactions start at about 350°C. The gasification reaction of volatile matters (VM) and char with steam is rather slow at such temperature. The concentration of volatiles outside the coal particle increases quickly, and gasification only takes place after devolatilization is accomplished. However, in case the heating rate is high, then both pyrolysis and gasification occur concurrently, so that high concentration of volatiles is never permitted to build up. This explains why a clean gas in such a short time is produced from high-temperature entrained-flow gasifiers. In Ref. [3], it is indicated that in contrast with a counter flow moving-bed process, where lump coal is employed, the rate of heating is slow and a high volatiles concentration grows up and discarded unreacted from the reactor by the syngas.
Volatiles Combustion
The devolatilization of coal produces multi species, which include tars, hydrocarbon liquids, and gases such as CH4, CO, CO2, H2, H2O, HCN, and so on. These react with the oxidant surrounding the coal particle. The extent to which the oxidant is completely or partially depleted depends on the quantities of volatiles produced [3].
In combustion, where there is excess of oxygen, the volatiles are completely combusted. The moderating effect of carbon dioxide and water vapour reduces the temperature. In gasification the recycled gas contains notable amounts of carbon monoxide and hydrogen (up to 90% for an oxygen-blown gasifier) which causes tremendously high local temperatures, in case it comes into contact with the oxidant [3].
Coal Gasification
Gasification commences under shortage of oxygen. Coal is first heated in a closed chamber where it undertakes a pyrolysis process at temperatures over 400°C. During pyrolysis, hydrogen rich VM is released, together with tar, phenols, and gaseous hydrocarbons. Then, char is gasified, with the liberation of gases, tar vapours, and solid residues. The ruling reactions subsist of partial oxidation of char, which produces a syngas with high fractions of H2 and CO. The process happens at temperatures between 800 and 1800°C. Exact operating conditions depend on coal type, properties of the resulting ash, and the gasification technology. The oxidant is the highly important variable in the gasification process. It can be either air or pure oxygen in case the process includes an air separation unit (ASU) for O2 production. The deployment of an ASU adds cost to the power plant.
If the coal is heated externally, the process is termed “allothermal,” while “autothermal” process assumes heating of coal by means of exothermal chemical reactions undergoing inside the gasifier. Oxygen and water molecules oxidize the coal and produce a gaseous mixture of CO2, CO, H2O vapour, H2, and CH4. Some by-products like tar and phenols are also possible end products, depending on the type of the employed gasification technology. The likely wanted end product is usually syngas (i.e., a combination of H2 + CO), but the released coal gas may be further refined to produce extra quantities of H2, according to: 3C (coal) + O2 + H2O → H2 + 3CO [4].
Char Gasification
As only char and ash left, the char particles sustain two important endothermic, heterogeneous gasification reactions: (1) the Boudouard reaction: C(s) + CO2 ↔ 2CO (or, more specifically, the reverse Boudouard reaction, and it is also known as carbon dioxide-char gasification), and (2) the C(s) + H2O (g) → CO + H2 or C(s) + 2H2O (g) → CO2 + 2H2 reactions (also named steam-char gasification), where s and g refer to solid and gas, respectively. Both reactions are endothermic [2].
The steam-char reaction is the supreme contributor to the production of both H2 and CO, which are the primary reactive constituents of the syngas [2].
Water-Gas Shift (WGS)Process Inside Gasifiers
The water-gas shift reaction is an equilibrium process: CO + H2O (g) ↔ CO2 + H2. The forward reaction is exothermic, in which CO and steam are converted to H2 and CO2. The forward reaction is energetic at temperatures less than 700°C. At higher temperatures, near 1000°C, the net reaction is slow and negligible. More than 1200°C, the backward reaction becomes commanding. The reaction rate of the WGS is usually slow without using catalysts; however, in the gasifier, the reaction rate is usually enhanced by the catalytic effect of metallic components in coal. In gasifiers which utilize the quench method to cool down the syngas to near 200°C, the residence time is very short to achieve any remarkable forward WGS reaction, despite that the equilibrium constant value is large at low temperatures, because the catalytic effect from metals in coal is feeble in the quenched syngas since most of the metals have become molten slag, which is extracted during the gasification process, before quenching takes place [2].
Methanation
The methanation reaction [C + 2H2 → CH4] is predominantly exothermic and pressure favourable, so it is generally inactive in high-temperature atmospheres, for instance in high-temperature entrained flow gasifiers [2].
Types of Gasifiers
Detailed descriptions of gasifier types and their operation can be found in many references. Operating data and innovative gasifiers studied prior to the 1980s are reported in some early studies. Recent approaches to gasification such as catalytic, molten salt, plasma, or secondary heated systems are dealt with elsewhere. Only the major gasifiers in use today are described. It is suggested that the foremost gasifier types are moving bed, fluidized bed, and entrained flow ones [5].
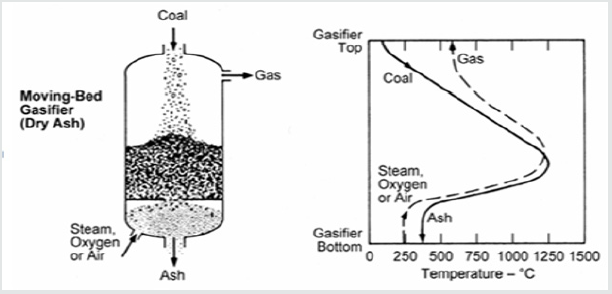
Fixed Bed Gasifier
A fixed bed gasifier is illustrated in Figure 3 The coal to ash and gas production schemes along the gasifier as the temperature varies is shown. These gasifiers are counter current flow ones; coal is fed at the top and the oxidant from the bottom. As the coal slowly proceeds down through the gasifier, it is gasified and the resulting ash drops out of the bottom. The counter current flow design allows the utilization of the heat of reaction from the gasification reactions to preheat the coal before its entrance to the gasification reaction zone. Consequently, the temperature of the syngas exiting the gasifier is remarkably less than the temperature required for total conversion of coal [6].
These features are notable in such gasifiers: the residence time of coal inside may be of the order of hours, low oxidant requirements, relatively high content of CH4 in the resulting gas, production of hydrocarbon liquids, such as oils and tars, high cold gas thermal efficiency when including the heating value of the hydrocarbon liquid, limited ability to handle fines, and special requirements for handling caking coal. Fixed bed gasifiers are still in use and have long industrial experience as the so named Lurgi type. However, reliable but are not suitable for one large scale gasifier. Recently a Lurgi of 1600 ton/day capacity was manufactured [7].
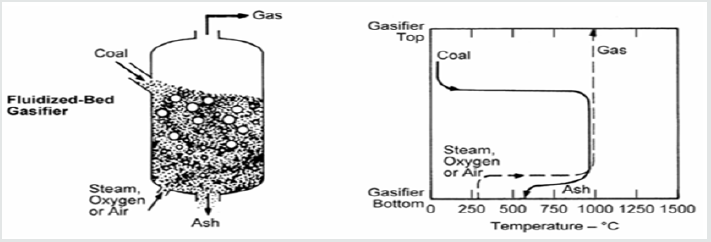
Fluidized Bed Gasifier
Figure 4 shows a fluidized bed gasifier. The figure illustrates how coal is converted into ash and the gas production across the gasifier as the temperature goes up. It is a back-mixed or wellstirred reactor where there is a consistent mixture of fresh coal particles mixed with older ones, some of which are partially gasified, and some are totally gasified. The mixing regime allows consisting temperatures throughout the bed. The gas flow into the reactor (oxidant, steam, recycled syngas) must be adjusted such that to suspend coal particles within the bed but not too high to entrain them out. As the gasified coal particles gets smaller and lighter, they will escape from the gasifier. In order to avoid particle agglomeration, it is important to ensure that the temperatures within the bed to be lower than the initial ash fusion temperature of coal [6].
A cyclone downstream the gasifier will capture the larger particles that are entrained out and return them back to the bed. The residence time of coal particles in these gasifiers is shorter than that in a moving bed one [6]. The main characteristics of these gasifiers are extensive solids recycling, uniform and moderate temperature, and moderate oxygen and steam requirements. Fluidized-bed gasifiers have been developed for the gasification of low-grade fuels or feed stock. The working principle of the fluidized bed embraces even distribution of oxidant through the reactor. Gas bubbles tend to flow via the less congested area, and this results in the existence of a dead zone inside the reactor. This creates difficulties in scaling up design and operation. Most distinguished fluidized-bed examples are fluidized bed boiler and waste pyrolysis plants [7].
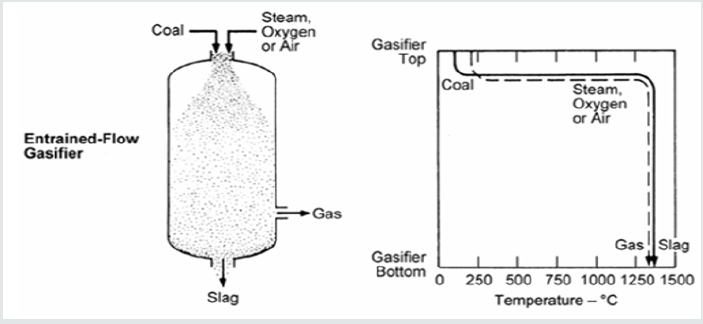
Entrained Flow Gasifier
Figure 5 shows a diagram of an entrained flow gasifier. The figure gives the coal gas conversion and gas production along the reactor as a function of temperature. Co-current flow of pulverized coal and oxidant are injected into the reactor. The coal is rapidly heated up and reacts with the oxidant. The residence time in these gasifiers is of the order of seconds. Because of this short time, such gasifiers must perform at high temperatures in order to attain high carbon conversion efficiency. Therefore, shows that the majority of entrained flow gasifiers employ oxygen rather than air and operate above the slagging temperature of coal [6].
Properties of entrained flow gasifiers include: high-temperature
slagging performance, entrainment of some molten slag in the
raw syngas, relatively large oxidant requirements, vast chunk of
sensible heat in the raw syngas, and the potential to gasify all coal
ranks, caking characteristics, or degree of fines.
The processes that need a high throughput capacity in a single
reactor generally use entrained bed type, as in IGCC, since the
reactor size can be reduced by the fast residence time (typically
less than 5 sec) as well as by high pressure. Although large scale
operation of entrained bed gasifiers have been successfully
operated commercially, however, the experience is not long enough
as in the case of fixed or fluidized bed gasifiers. It is concluded
that the foremost disadvantage of entrained bed gasifier is its high
capital cost due to the compact configuration of parts [7].
Concluding Remarks on Gasifiers
From the above discussions it is concluded that the favourable is the entrained flow one because:
a) The residence time is too short and in the order of second
so it must operate at elevated temperatures to attain high
carbon conversion efficiency.
b) It produces a clean gas in a short time as a result of
operating at high temperature.
c) Large scale performance due to the short residence time
and high carbon conversion efficiency, thus increasing both the
throughput coal and yield abundantly.
d) It increases the gas velocity which can reach 80–100 m/s.
e) The manufacturing cost is less compared to other types
of gasifiers.
f) Low hydrocarbon because of the high temperature.
g) High CO2 capture because of the high CO in syngas.
h) None or very little tar formation because it is a downdraft
gasifier.
i) The radiant syngas cooling can increase the cycle
efficiency by 4 – 5% over full quench types.
Gasifiers need further improvements and developments in order to increase their efficiencies and performance for more availability of IGCC systems.
Methodology of Calculations
During the gasification of solid carbon, whether in the form of coal, coke, or char, the main chemical reactions are those associated with C, CO, CO2, H2, H2O (or steam), and CH4. These reactions are:
Combustion reactions
C + ½O2 = CO - 111 MJ/kmol (1)
CO + ½O2 = CO2 - 283 MJ/kmol (2)
H 2 + ½O2 = H2O - 242 MJ/kmol (3)
The Boudouard reaction
C + CO2 = 2CO + 172 MJ/kmol (4)
The water gas reaction
C + H2O = CO + H2 + 131 MJ/kmol (5)
The methanation reaction
C + 2H2 = CH4 + 75 MJ/kmol (6)
As reactions with free oxygen are all complete under gasification conditions, reactions (1), (2) and (3) do not need to be considered in determining an equilibrium syngas composition. The three heterogeneous (i.e. gas and solid phase) reactions (4), (5) and (6) are sufficient. In general, we are concerned with situations where the carbon conversion is also essentially complete. Under these circumstances, Equations (4), (5), and (6) can be reduced to these two homogeneous gas reactions:
The water gas shift reaction
CO + H2O = CO2 + H2 + 41 MJ/kmol (7)
The steam methane reforming reaction
CH4 +H2O = CO + 3H +206 MJ/kmol (8)
Note that by subtracting the moles and heat effects from reaction (4) from those in reaction (5), one obtains reaction (7), and by subtracting reaction (6) from (5), one obtains reaction (8). Thus reactions (7) and (8) are implicit in reactions (4), (5), and (6) – but not the other way around. Three independent equations always contain more information than two. Reactions (1), (4), (5), and (6) describe the four ways in which a carbonaceous or hydrocarbon fuel can be gasified. Reaction (4) is important for the production of pure CO when gasifying pure carbon with an O2/CO2 mixture. Reaction (5) plays a predominant role in the water gas process. Reaction (6) is the basis of all hydrogenating gasification processes. But most gasification processes rely on a balance between reactions (1) (partial oxidation) and (5) (water gas reaction). For real fuels (including coal, which also contains hydrogen), the overall reaction can be written as:
CnHn + n/2 O2 = nCO + m/2 H2 (9)
Where
- For gas, as pure methane, m = 4 and n = 1, hence m/n = 4
- For oil, m/n = 2, hence m = 2 and n = 1
- For coal, m/n = 1, hence m = 1 and n = 1.
Gasification temperatures are so high that, thermodynamically
as well as in practice, no hydrocarbons other than methane can be
present in any appreciable quantity.
Thermodynamic equilibrium: As an example: Equations (1) and (5) produce H2 and CO which absorbs and releases heat
C + ½O2 = CO - 111 MJ/kmol (10)
C + H2O = CO + H2 + 131 MJ/kmol (11)
The molar weight and mass flow rate ton/day (TPD) of each
component in both sides of Eqs. (10) and (11) can be determined
and written under each equation for clarity.
Equation (10) releases heat, and Eq. (11) absorbs heat. The
heat released is used to heat a fire tube boiler or utilized in another
gasification application.
The above methodology is used in what follows to conduct
detailed calculations of the syngas composition, cold gas efficiency,
and carbon conversion efficiency, but for one coal type, bituminous
coal, which is the mostly used in power plants. The purpose of this
is to show the step by step of such calculation procedures. This is
done for the three studied gasification processes. The results for
other coals are given briefly following the same procedures as in
the case of bituminous coal.
Data and Assumptions
To perform the calculations, numerical data and assumptions should be made. The coal ranks considered cover the high grade ones (anthracite and bituminous) and low ranks (sub-bituminous and lignite). Mass percentages of constituents of coals are depicted in Table 1 [5].
To get coal chemical compositions, we must calculate mole
fractions of all species and hence their normalized values with
respect to carbon, and these are presented in Table 2. Coal
compositions as extracted from the normalized mole fractions, in
Table 2, are shown in Table 3.
Equations for complete combustion of coals are indicated in
Table 4.
Table 2: Mole fractions and normalized values with respect to carbon of coal constituents.
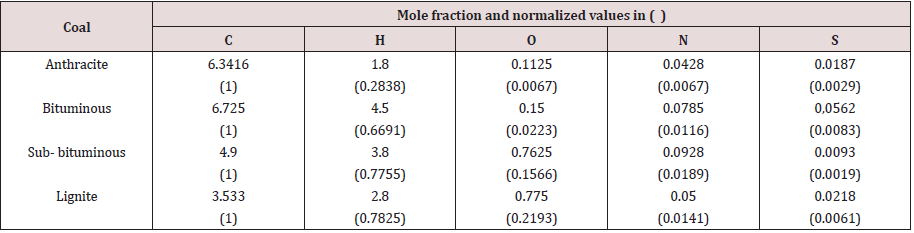
Note: Molar fractions are estimated by dividing the mass percentage of each species (from Table 1) by its atomic weight. Example: for bituminous coal, the mole fraction of C= 76.1/12= 6.6735, H= 4.5/1= 4.5, S= 1.8/32= 0.0083. Normalized values are obtained by dividing the mole fraction of the species by that of carbon. For example: for bituminous coal, C= 7.625/7.625= 1, and N= 0.0785/7.625= 0.0116.
Table 3: Chemical compositions of coal ranks. Note: coal compositions are extracted from the normalized values given in Table 2.
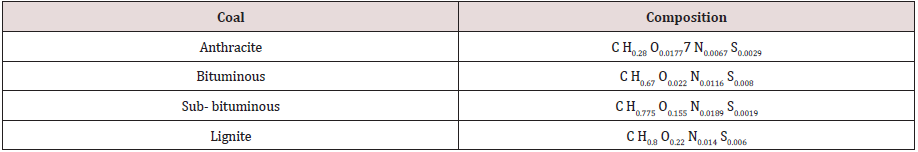
Table 4: Equations for complete combustion of coals.
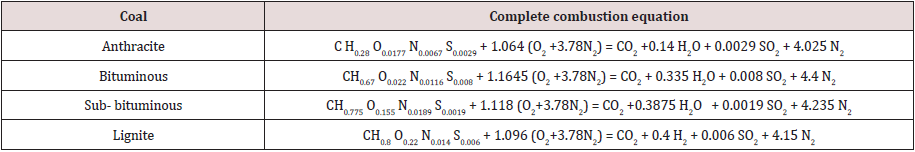
The heating values used in calculations of cold gas efficiencies are listed in Table 5.
Table 5: Heating values of coals and gases.
Note: HHV= High Heating Value, and LHV= Low Heating Value.
For all present calculations, we assumed a flow rate of coal in gasifiers= 2500 TPD. Oxygen is blown in gasifiers and the pressure=
30 atm.
The chemical equation in gasifiers is
CH0.67 O0.022 N0.0116 S0.008. +a H2O + b O2 = e CO + f H2 + g COS + j H2S + k CO2 + l CH4 + m H2O
Where: a, b, c…..etc are fractions determined for each coal and each gasifier.
Detailed Calculations for Bituminous Coal
In the following calculations the molar weight of carbon in coal Mc= 100/6.725= 14.869 gm/mole. From the mass weights in Table 1, moisture and ash flow rates are 82.5 (= 0.033x2500) and 155 TPD (= 0.062x2500), respectively.
Entrained Flow Gasification
Reactions in gasifier:
A. Pyrolysis and devolatilization of coal
Cs = 0.3345 H2 + 0272 H2O+ 0.0058 N2 + 0.008H2S + 0.0115 O2 (12)
14.869 0.669 0.4896 0.1624 0.272 0.3568
2500 TPD 112.5 82.5 27.5 47.812 60
H2 + S = H2S (13)
2 32 34
2.812 TPD 45 47.812
B. Gasification
3Cs + H2O + O2 = 3CO + H2 (14)
44.607 18 32 84 2
2500 TPD 1008.81 1793.44 4707.781 112.09
C. Combustion
CO + 0.5 O2 = CO2 (15)
28 16 44
536.48 TPD 306.56 843.04
D. Water from partial combustion
H2 + 0.5 O2 = H2O (Water with slag) (16)
2 16 18
7.5 TPD 60 67.5
From above calculations, ṁO2= 2100 TPD (= 84% by weight of coal), and ṁCO= 1008.81 TPD (=40.352% of slurry feed)
Mass flow rates of gasses in syngas (ṁgas) TPD
ṁCO2= 843.04 ṁCO= 4171.301 ṁH2= 214.278 ṁN2= 27.5 ṁH2S= 47.812
These mass flow rates are used to determine the CGη.
CH0.67 O0.022 N0.0116 S0.008. + 0.333 H2O + 0.39 O2 = 0.886 CO + 0.637 H2 + 0.008 H2S + 0.114 CO2 + 0.006 N2+ 0.022 H2O (17)
Total moles of syngas = Σ fractions in the RHS of Eq. (17) = 1.673
Ygas (%) = (number of moles in species / total moles in syngas) x 100
YCO2= 6.814 YCO= 52.958 YH2= 38.075 YN2= 0.358 YH2S= 0.478
Cold gas efficiency (CGη)
CGη is estimated by considering only H2, CO, and CH4 in syngas.
∆HC = 300088.4 {= (214.278x1000 / 24x60x60) x 121000} + 487375.97 = 787464.37 kW
CGη = [∆Hc / (ṁc (= 2500 TPD) × HHV) Coal] ×100 = [787464.3 / 963541.67 (= {(2500x1000/24x60x60) x 333000}] ×100= 81.726 %
Carbon conversion efficiency (CCη)
CCη = ṁ carbon in coal / ṁ carbon in syngas = [(2500 / 2500) × 100 = 100 %
Fluidized Bed Gasification
Reactions in gasifier:
A. Pyrolysis and devolatilization: Equation (12) still applies.
B. Gasification and combustion
3CS + H2O + O2 = 3CO + H2 (18)
44.607 18 32 84 2
2265.2 TPD 914.062 1625 4265.625 101.562
Water from partial combustion: Equation (16) is the same.
Water gas shift
CO + H2O = CO2 + H2 (19)
28 18 44 2
911.459 TPD 585.938 1432.293 65.104
Methanation
CS + 2H2 = CH4 (20)
14.869 4 16
159.8 TPD 42.988 171.955
The above results reveal that ṁO2 and ṁsteam (steam feed) are 1750 and 1500 TPD, i.e. 70 and 60% by weight of coal, respectively.
ṁgas (TPD)
ṁCO2= 1432.293 ṁCO= 3354.166 ṁH2= 228.678 ṁN2= 27.5 ṁCH4= 171.955 ṁSteam= 82.5
CH0.67 O0.022 N0.0116 S0.008. + 0.495 H2O + 0.302 O2 = 0.712 CO + 0.68 H2 + 0.064 CH4 + 0.193 CO2 + 0.027 H2O + 0.006 N2 (21)
Ygas (%):
Total moles of syngas = 1.682
YCO2= 11.474 YCO= 42.33 YH2= 40.428 YCH4= 3.805 YN2= 0.356 YH2S= 0 YSteam= 1.605
CGη
∆HC = 320255.07 + 391901.69 + 99501.044 = 811657.8 kW
- CGη = [∆HC / (ṁ × HHV) Coal] × 100 = [811657.8 / 963541.67] ×100= 84.237 %
CCη
CCη = = [2425 / 2500] × 100 = 97%
Fixed Bed Gasification
Reactions in gasifier:
A. Pyrolysis and devolatilization: Equations (12) and (13)
are applicable.
B. Gasification and combustion
3CS + H2O + O2 = 3CO + H2 (22)
44.607 18 32 84 2
1847.008 TPD 745.312 1325 3478.125 82.812
C. Water from partial combustion: Equation (16) applies
D. Steam Gasification
CS + 2H2O = CO2 + 2H2 (23)
14.869 36 44 4
105.193 TPD 254.688 311.285 28.298
Direct methanation
CS + 2H2 = CH4 (24)
14.869 4 16
497.799 TPD 133.916 535.663
Here ṁO2 and ṁsteam (steam feed) are 1325 and 1000 TPD, i.e. 53 and 40% by weight of coal, respectively.
ṁgas (TPD)
ṁCO2= 311.285 ṁCO= 3478.125 ṁH2= 79.38 ṁN2= 27.5 ṁCH4= 535.663 ṁH2 = 47.812 ṁSteam= 82.5
CH0.67 O0.022 N0.0116 S0.008. + 0.33 H2O + 0.246 O2 = 0.739 CO + 0.236 H2 + 0.199 CH4 + 0.008 H2S + 0.042 CO2 + 0.006 N2 + 0.027 H2O (25)
Ygas (%):
Total moles of syngas = 1.257
YCO2= 3.341 YCO= 58.79 YH2= 18.775 YCH4= 15.831 YN2= 0.477 YH2S= 0.636 YH2O= 2.148
CGη
∆HC = 111171.55 + 406384.51 + 309959.16 = 827515.22 kW
CGη = [∆HC/ (ṁ × HHV) Coal] × 100 = [827515.22 / 963541.67] × 100= 85.882 %
CCη
CCη = = [2450 / 2500] × 100 = 98 %
Calculations for Other Coals
Entrained Flow Gasification
Anthracite coal
Mass flow rate of oxygen blown in gasifier (ṁO2) = 1980.297 TPD
Mass flow rate of water slurry (ṁwater) = 951.25 TPD
ṁgas (TPD):
ṁCO2= 795.262 ṁCO= 3933.091 ṁH2= 144.132 ṁN2= 15 ṁH2S= 15.938 ṁSteam = 135
Chemical equation:
CH0.28 O0.0177 N0.0067 S0.0029 + 0.333 H2O + 0.39 O2 = 0.886 CO + 0.454 H2 + 0.003 H2S + 0.114 CO2 + 0.003 N2+ 0.047 H2O (26)
Ygas (%):
All moles of syngas = 1.507
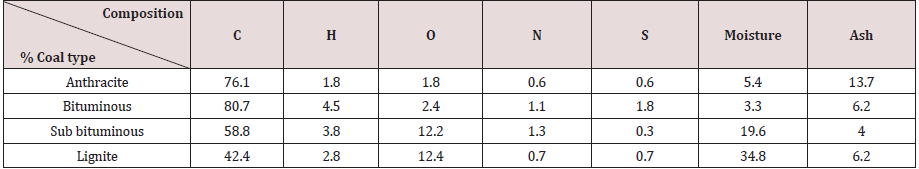
YCO2= 7.564 YCO= 58.792 YH2= 30.126 YN2= 0.2 YH2S= 0.2 YH2O= 3.118
Sub bituminous coal:
ṁO2 = 1530.112 TPD
ṁwater = 735 TPD
ṁgas (TPD):
ṁCO2= 614.473 ṁCO= 3038.971 ṁH2= 138.073 ṁN2= 32.5 ṁH2S= 7.969 ṁSteam = 490
Chemical equation:
C H0.775 O0.155 N0.0189 S0.0019 + 0.333 H2O + 0.39 O2 = 0.886 CO + 0.563 H2 + 0.002 H2S + 0.114 CO2 + 0.01 N2+ 0.222 H2O (27)
Total moles of syngas = 1.797
YCO2= 6.344 YCO= 49.304 YH2= 31.33 YN2= 0.556 YH2S= 0.111 YH2O= 12.354
Lignite coal:
ṁO2 = 1103.346 TPD
ṁwater = 530 TPD
ṁgas (TPD):
ṁCO2= 443.09 ṁCO= 2191.367 ṁH2= 89.045 ṁN2= 17.5 ṁH2S= 18.594 ṁSteam = 870
Chemical equation:
C H0.8 O0.22 N0.014 S0.006 + 0.333 H2O + 0.39 O2 = 0.886 CO + 0.504 H2 + 0.006 H2S + 0.114 CO2 + 0.007 N2+ 0.547 H2O (28)
Ygas (%):
Total moles of syngas = 2.064
YCO2= 5.523 YCO= 42.926 YH2= 24.418 YN2= 0.339 YH2S= 0.29 YH2O= 26.502
Fluidized Bed Gasification
Anthracite coal:
(ṁO2) = 1650.248 TPD
(ṁwater) = 1414.498 TPD
ṁgas (TPD):
ṁCO2= 1350.649 ṁCO= 3162.975 ṁH2= 156.040 ṁN2= 15 ṁCH4= 162.007 ṁSteam = 135
Chemical equation:
C H0.28 O0.0177 N0.0067 S0.0029 + 0.495 H2O + 0.325 O2 = 0.712 CO + 0.492 H2 + 0.064 CH4+ 0.193 CO2 + 0.003 N2+ 0.047 H2O (29)
Ygas (%):
Total moles of syngas = 1.511
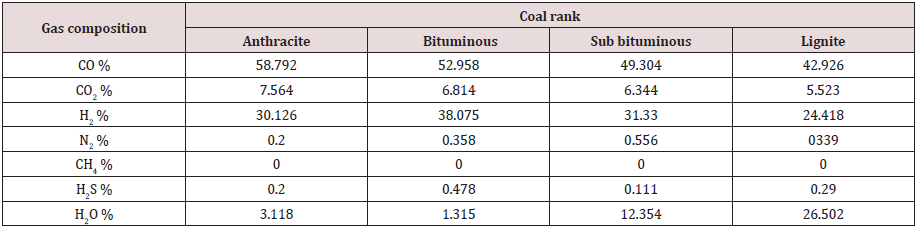
YCO2= 12.773 YCO= 47.121 YH2= 32.561 YN2= 0.198 YCH4= 4.235 YH2O= 3.11
Sub bituminous coal:
ṁO2 = 1275.093 TPD
Mass flow rate of steam for gasification (ṁsteam) = 1092 TPD
ṁgas (TPD):
ṁCO2= 1043.603 ṁCO= 2443.928 ṁH2= 147.018 ṁN2= 32.5 ṁCH4= 125.178 ṁSteam = 490
Chemical equation:
CH0.775 O0.155 N0.0189 S0.0019 + 0.495 H2O + 0.325 O2 = 0.712 CO + 0.6 H2 + 0.064 CH4+ 0.193 CO2 + 0.01 N2+ 0.222 H2O (30)
Ygas (%):
Total moles of syngas = 1.801
YCO2= 10.716 YCO= 39.533 YH2= 33.314 YN2= 0.555 YCH4= 3.553 YH2O= 12.326
Lignite coal:
ṁO2 = 919.455 TPD
ṁwater = 788.104 TPD
ṁgas (TPD):
ṁCO2= 752.53 ṁCO= 1762.288 ṁH2= 96.251 ṁN2= 17.5 ṁCH4= 90.264 ṁSteam = 870
Chemical equation:
CH0.8 O0.22 N0.014 S0.006 + 0.495 H2O + 0.325 O2 = 0.712 CO + 0.544 H2 + 0.064 CH4+ 0.193 CO2 + 0.007 N2+ 0.547 H2O (31)
Ygas (%):
Total of moles of syngas = 2.067
YCO2= 9.337 YCO= 34.446 YH2= 26.318 YN2= 0.338 YCH4= 3.096 YH2O= 26.463
Fixed Bed Gasification
Anthracite coal:
ṁO2 = 1249.473 TPD
ṁsteam = 942.999 TPD
ṁgas (TPD):
ṁCO2= 293.541 ṁCO= 3279.868 ṁH2= 16.97 ṁN2= 15 ṁCH4= 504.981 ṁSteam = 135 ṁH2S= 15.938
Chemical equation:
CH0.28 O0.0177 N0.0067 S0.0029 + 0.33 H2O + 0.246 O2 = 0.739 CO + 0.053 H2 + 0.199 CH4+ 0.042 CO2 + 0.003 N2+ 0.047 H2O + 0.003 H2S (32)
Ygas (%):
Total moles of syngas = 1.086
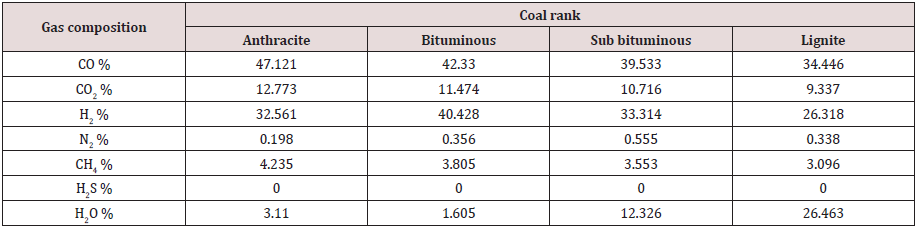
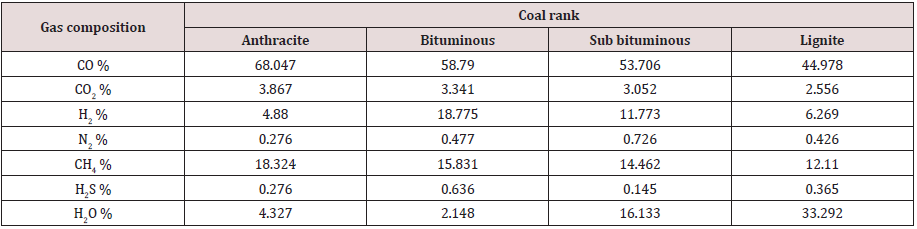
YCO2= 3.867 YCO= 68.047 YH2= 4.88 YN2= 0.276 YCH4= 18.324 YH2O= 4.327 YH2S= 0.276
Sub bituminous coal:
ṁO2 = 965.427 TPD
ṁsteam = 728.624 TPD
ṁgas (TPD):
ṁCO2= 226.81 ṁCO= 2534.247 ṁH2= 39.819 ṁN2= 32.5 ṁCH4= 390.183 ṁSteam = 490 ṁH2S= 7.969
Chemical equation:
CH0.775 O0.155 N0.0189 S0.0019 + 0.33 H2O + 0.246 O2 = 0.739 CO + 0.162 H2 + 0.199 CH4+ 0.042 CO2+ 0.01 N2+ 0.222 H2O + 0.002 H2S (33)
Ygas (%):
Total moles of syngas = 1.376
YCO2= 3.052 YCO= 53.706 YH2= 11.773 YN2= 0.726 YCH4= 14.462 YH2O= 16.133 YH2S= 0.145
Lignite coal:
ṁO2 = 696.159 TPD
ṁsteam = 525.403 TPD
ṁgas (TPD):
ṁCO2= 163.55 ṁCO= 1827.416 ṁH2= 18.195 ṁN2= 17.5 ṁCH4= 281.356 ṁSteam = 870 ṁH2S= 18.594
Chemical equation:
CH0.8 O0.22 N0.014 S0.006 + 0.33 H2O + 0.246 O2 = 0.739 CO + 0.103 H2 + 0.199 CH4+ 0.042 CO2+ 0.007 N2+ 0.547 H2O + 0.006 H2S (34)
Ygas (%):
Total moles of syngas = 1.643
YCO2= 2.556 YCO= 44.978 YH2= 6.269 YN2= 0.426 YCH4= 12.11 YH2O= 33.292 YH2S= 0.36
Results and Discussion
Syngas Compositions Produced from Gasifiers
Partial combustion of coal inside the gasifier produces raw syngas consisting of different gases. Some of these are re-burned again in the turbine, such as CO, H2, and CH4. Some are disposed off, including H2S. Some species will withdraw heat from the system, such as N2 and water. Some gases such as CO2 are harmful to the environment. Tables 6, 7, and 8 give the raw syngas compositions, as obtained from the above calculations, for all coal ranks, for the three studied gasifiers.
The numbers in Tables 6, 7, and 8 indicate the mole percentage of each gas component produced in the syngas by the partial combustion of coal. The mass ratio of each gas was not calculated, and it was sufficient to adopt the mole percentage values because they are more precise in expressing the gas composition since the mole mass for each gas species is different.
As seen the main components of the syngas are the two reactive species H2 and CO in addition to some CH4 and CO2. The syngas composition depends significantly, as show in Tables 6, 7, and 8, on the gasification process and the coal rank. Each gasification technology has its own gasifier design with a specific chemical reaction and operating conditions. Coals have different constituents and chemical and physical properties. All these justify dependence of syngas composition on the gasifier type and coal rank. Syngas composition depends on other factors, such as coal preparation and its particle size, coal and gas residence time in the gasifier, coal feeding procedure whether dry or slurry, heating rate, flow directions, method of mineral removal (dry ash or slag), heat generation source, and operating temperature and pressure. No one gasifier or coal type will satisfy the desired application or syngas composition and it is a matter of compromise and trade off. It is seen from the above results that the entrained flow gasifier produces a satisfactory syngas composition with varying values for different coal ranks.
The main aim of combusting syngas is the production of heat and to result in less emissions. To generate high combustion, heat the fed syngas to the gas turbine should be rich in H2 and CO in addition to light hydrocarbons. The syngas should contain minimum or no CH4 and sulphur in order to reduce emissions. Less sulfur means significantly lower SOx emissions than that from conventional power systems.
The composition of syngas immensely influences the emission levels. The combustion of H2 and CO gases result in higher combustion temperature which favours thermal NOx formation. NOx can be either thermal (30%) or fuel (70%). However, such higher temperatures lead to complete combustion and hence reduces emissions of organic volatile matter which are traces of hydrocarbons in the syngas. NOx formation is a concerning issue in burning fuels whether fossil or syngas. To reduce fuel NOx, which constitutes the higher percentage of the total NOx, the nitrogen content in syngas should be small in addition to reducing the contact time between the fixed N2 in fuel and O2 in the combustion air. Various technologies are already available to reduce thermal NOx, e.g. flue gas recirculation (FGR) and staged combustion which reduces also fuel NOx.
Syngas combustion is greatly affected by the content of H2 in it. The burning velocity increases with the existed amount of H2 because the density is much less compared with that of natural gas. Also increasing the H2 content in syngas increases the flame stability. The presence of CH4 in syngas reduces the peak flame temperature but increases the prompt NO significantly.
The raw syngas produced from gasifiers needs a cleaning system in case used as fuel for a gas turbine in an IGCC plant. Cleaning syngas means the removal of particulate matter, metallic compounds, and other undesirable pollutants such as sulphur. The cleaning system may be pre-combustion or post-combustion. A water gas shift system may be included to enhance the H2 content in the gas. A CO2 capture system can be employed if desired. Therefore, IGCC plants are more environmentally cleaner than current ones especially if CO2 capture system is adopted.
It should be realized that there are many technological problems which should be encountered for a successful IGCC technology. The most important issue is the gas turbine which is the core of a combined cycle system. The differences in the properties between syngas and NG dictate new considerations in the design of the gas turbine in an IGCC system. For instance, the lower calorific value of syngas fuels requires a significant increase in the mass flow rate of fuel supply to the gas turbine as compared with burning NG. As a result the output power will increase and this needs a different design of the turbine to allow for the increased flow rate. Also the higher content of H2 in syngas which has a higher flame velocity may lead to difficulties in controlling the combustion mechanism. Also, syngas combustion is different from NG because of the presence of H2 and CO which have higher adiabatic flame temperatures than CH4. So, new issues should be looked after for a proper reliable gas turbine performance.
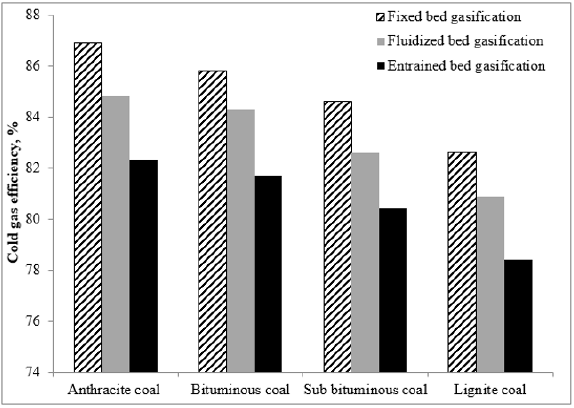
Cold Gas Efficiency
le 9 exhibits the CGη and CCη for the different gasifiers and coals. Figure 6 lists some cold gas efficiencies for various gasification processes for all coal ranks. The cold gas efficiency is the ratio of fuel heat content to the syngas heat content at ambient conditions and is a measure of how efficiently fuel energy is converted into syngas energy. For the cases reported, the fixed bed is most efficient but has the disadvantage that some of the syngas energy is produced in tars. The listed efficiencies (~86%) support the rule of thumb that approximately 15% of the feedstock heating value is used to convert the feedstock to syngas.
Table 9: Coal gasification (CGη) and coal capture (CCη) efficiencies for different gasifiers and coal ranks.
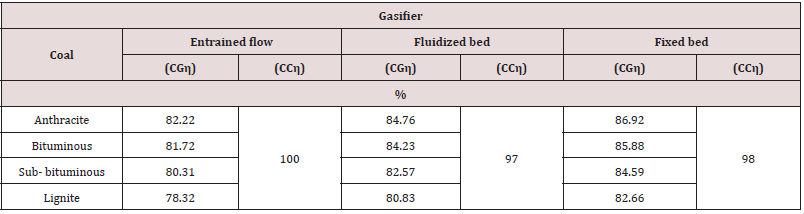
The results depict that the entrained flow gasifier, for all coal ranks, is the predominant one because of its low cold gas efficiency which means low loses in coal fired because of the high temperature in the gasifier, higher carbon conversion efficiency (100%), low tar, and the low methane produced which means lower emission. The second good, for all coal types, is the fluidized bed gasifier.
Conclusions
Irrespective of new discoveries of natural gas reserves and new techniques (such as hydraulic fracturing or fracking) being developed to increase cheaper natural gas production, coal will continue to be a major energy source to produce electricity in the world, either for economic reasons or as a strategy to safeguard national energy security and independence. The conventional way of burning coal is environmentally unfriendly; therefore, it is essential that cleaner methods of utilizing coal be developed. IGCC is one of the most promising methods to generate electricity in a more efficient, environmentally friendly manner than conventional fossil fuel plants. Example step by step manual calculation procedure was conducted for one coal (bituminous).
Syngas composition depends incredibly on the gasification process and coal type. Each gasification technology has its own specific chemical reaction and operating conditions. The entrained flow gasifier satisfies most of the requirements needed for the appropriate syngas for IGCC plants, for all coals. This gasifier produces raw syngas with zero CH4 , high H2 and CO, and low CO2, H2O, N2, and H2 S.
The entrained flow gasifier is the most viable of the three
because of its high carbon conversion efficiency which means low
loses in coal fired and low cold gas efficiency, high temperature in
the gasifier, low tar, and low methane produced resulting in low
emissions, and it produces a clean gas in such a short time because
of the employed high temperature. Compare this with a countercurrent
fixed-bed process, which uses lump coal, the heating up
rate is slow with a built up of high volatiles concentration that are
removed unreacted from the reactor by the syngas.
For the technology of IGCC plants to be competitive and
economically feasible, their availability should be increased and the
capital cost to be reduced. Achieving these, IGCC systems will be
fully commercial and of increased widespread use (table 10).
Conflict of Interest
The authors declare that there is no conflict of interest regarding publication of this paper.
References
- Rafael Luque, James G Speight (Eds) (2015) Gasification for synthetic fuel production: Fundamentals, processes, and applications. Woodhead Publishing Series in Energy, Cambridge, England.
- Ting Wang, Gary J Stiegel (Eds) (2016) Integrated gasification combined cycle (IGCC) technologies, 1st Woodhead Publishing, Cambridge, England.
- Christopher Higman, Maarten vanderBurgt (2003) Gasification. Second Edition. Elsevier Science Publisher, Amsterdam, Netherlands.
- Yatish TShah (2017) Chemical energy from natural and synthetic gas. Florida: CRC Press. Taylor and Francis group publisher.
- Tim Lieuwen, Vigar young, Richard Yetter (Eds) (2009) Synthesis gas combustion fundamentals and applications, 1st CRC Press, Taylor and Francis group publisher, Florida, USA.
- Jeffrey Phillips (2006) Different types of gasifiers and their integration with gas turbines: The Gas Turbine Handbook. US Department of Energy Publisher, Washington DC, USA.
- Yong seung Yun (Ed) (2012) Gasification for practical applications. 1st Intech Open Publisher, London, United Kingdom.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...