Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5779
Review Article(ISSN: 2690-5779) 
Corrosion Protection of Aluminium in Sulphur dioxide Environment by the use of Nanocoating and Electrospray Techniques Volume 1 - Issue 3
Dr. Rajesh Kumar Singh1* and Jay Prakash Singh2
- 1Associate Professor, Department of Chemistry, Jagdam College, J P University, India
- 2Research Scholar, Department of Chemistry, Jagdam College, J P University, India
Received: August 27, 2020 Published: September 24, 2020
Corresponding author: Rajesh Kumar Singh, Department of Chemistry, Jagdam College, J P University, India
DOI: 10.32474/JOMME.2020.01.000112
Abstract
Aluminium is a very useful metal. It is easily converted into suitable forms so it fits for in various appliances like households, industries, aerospace, electronic, transport and other processes. It is produced corroding affect in sulphur dioxide environment. Al interacts with sulphur dioxide in presence of humidity to produce corrosive medium for its. It creates acidic environment for Al metal and forms corrosion cell on its surface. The electrode potential difference occur between Al|Al3+ anode and cathode H+|H2 thus corrosion reaction takes place on the surface of base metal and produces different forms corrosion like galvanic, pitting, stress, crevice, intergrannular etc. The organic compound terahydro-dibenzo[a,d][7]annulene-5,11-dioxime and zinc oxide electrospray were used for corrosion protection of Al in sulphur dioxide environment. The terahydro-dibenzo[a,d][7]annulene-5,11-dioxime and ZnO electrospray formed composite barrier on aluminium metal that barrier produced a passive layer for sulphur dioxide. The corrosion rate of uncoated and coated aluminium studied in different temperatures, concentrations, humidity and duration of days. The corrosion rate of metal was determined by gravimetric and potentiostat techniques. The surface adsorption phenomenon of coating compound terahydro-dibenzo[a,d][7]annulene-5,11-dioxime and zinc oxide electrospray was studied by Langmuir, Freundlich and Temkin isotherm and Arrhenius equation. The composite barrier formation on the surface of aluminium by terahydro-dibenzo[a,d][7] annulene-5,11-dioxime and electrospray zinc oxide was studied by activation energy, heat of adsorption, free energy, enthalpy and entropy. The surface coverage area and percentage coating efficiency of coating and electrospray compounds results found to be high in sulphur dioxide medium. Both compounds developed composited in sulphur dioxide to be stable and also enhanced durability of aluminium metal.
Keywords: Corrosion of aluminium; composite barrier; electrospray; sulphur dioxide etc.
Introduction
Corrosion is a surface phenomenon [1]. Materials corrode in hostile medium [2] and change occur their internal [3] and external morphology [4]. There are several remedies applied [5] to control the corrosion of materials [6] as per their environmental conditions [7]. There are several methods applied for corrosion protection of metals like metallic [8] and nonmetallic coatings [9], polymeric coating [10], inhibitors [11] and nano-coating [12]. Metallic coating [13] is related to electroplating, hot dipping, flame sprayed; vapour deposition, chemical conversion and diffusion [14]. Surface treatment used to check the corrosion of materials [15]. Various types of inorganic and organic inhibitors used to control the corrosion of metals in hostile medium [16]. The organic compounds acyclic, cyclic, aromatic, heterocyclic possessed primary, secondary, tertiary, quaternary amines, thiol, thioether, oxime, hydrazone, semicarbazone, phenylhydrazone functional groups [17] to decrease corrosion of metals in acidic medium. These functional groups formed thin barrier on metals surface and reduce the concentration of H+ ions [18]. The salts of inorganic compounds contain phosphorous applied for corrosion control. Polymeric coating provide protective barrier in corrosive medium [19]. Organic paint can be used to minimize corrosion in acidic medium [20]. Nano-coating methods applied for corrosion protection of metals by the use of chemical vapour deposition, nozzle spray, plasma spray and laser spray [21]. The composite barrier developed by the application of organic compounds and oxide of active metals.
Experimental
Weight loss experiment: The test samples of Al cut into (3X5X0.1) cm2 and rubbed with emery paper. The use of samples washed with acetone and dry with hot air gun. The samples were kept into 250 PPM sulphur dioxide environment. The corrosion rate of sample calculated without coating and with coating tetrahydrodibenzo[ a,d][7]annulene-5,11-dioxime and ZnO electrospray at 283, 293, 303, 313 and 323 K temperatures and that temperature recorded time 10, 20, 30, 40 and 50 days. The concentration of coating tetrahydro-dibenzo[a,d][7]annulene-5,11-dioxime and ZnO electrospray compounds used 30 mM and 10 mM by the used weight loss method.
Potentiostat
The 173 model EG & PG Princeton used to determine corrosion potential, corrosion current and corrosion current density. The electrochemical cell formed when Al sample kept between calomal auxiliary electrode and Pt electrode as reference electrode. The corrosion potential obtained absence and presence of tetrahydrodibenzo [a,d] [7] annulene-5,11-dioxime and ZnO electrospray.
The used organic compound was synthesized in laboratory and its method expressed as:
Synthetic method of terahydro-dibenzo [a,d] [7] annulene- 5,11-dioxime coating compound: Indanone poured into PCl5 at 0oC temperature in benzene solvent and reaction mixture was stirred two hours to produce 3-chloro-1H-indene. It used with potassium t-butaoxide in THF solvent to give 2, 3-didehydro-1H-indene. It trapped with cyclohexene to yield 2, 3-cyclohexane-indene. It oxidized with NaIO4 and RuO2 in solvent methylnitrile, carbon tetrachloride and water to obtain tetrahydro-dibenzo[a,d][7] annulene-5,11-dione. It heated with NH2OH with HCl, C2H5OH and pyridine solvent. The reaction mixture refluxed two hours to yield tetrahydrodibenzo[a,d][7]annulene-5,11-dioxime. The chemical reactions were written as
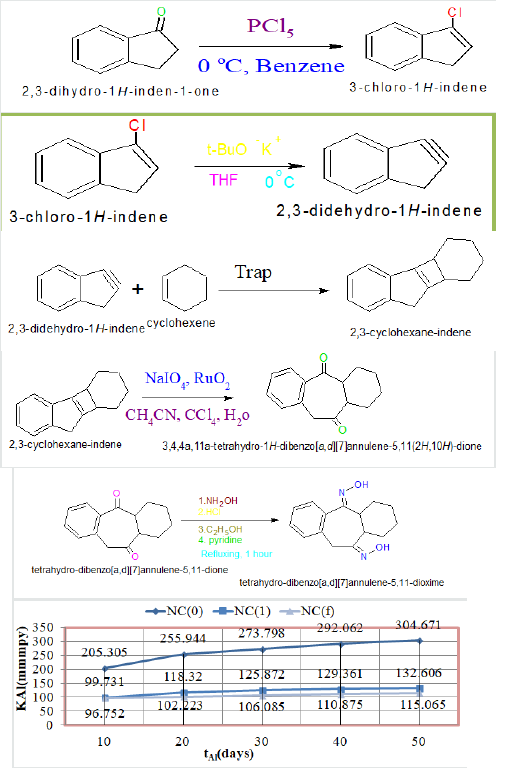
The corrosion rate of Al with time in SO2 environment: Its
corrosion rate was calculated by weight loss equation KAl(mmpy)
= 13.56 WAl / DAl AAl tAl (where WAl = weight loss of test coupon
expressed in kg, AAl = Area of test coupon in square meter, DAl =
Density of the material in kg. m-1, tAl = exposure time in hours)
in absence and presence of tetrahydro-dibenzo[a,d][7]annulene-
5,11-dioxime and ZnO electrospray and plot between K versus t in
(Figure 1). The corrosion rate of Al obtained at 10, 20, 30, 40 and
50 days with nanocoating of tetrahydro-dibenzo[a,d][7]annulene-
5,11-dioxime and ZnO electrospray and their values were written
in Table1. The corrosion rates indicated that corrosion rate of Al
reduced with coating and electrospray metals such trends clearly
noticed in (Figure 1).
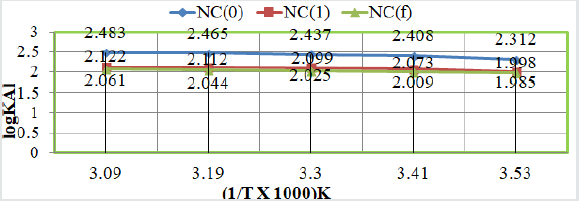
Corrosion rate of Al with temperature in SO2 environment:
The corrosion rates Al obtained without coating of tetrahydrodibenzo[
a,d][7]annulene-5,11-dioxime and electrospray ZnO and
their values were given in (Table 1). (Figure 2) plotted between logK
versus 1/T depicted that corrosion rate increased without coating
but its values decreased after coating of tetrahydro-dibenzo[a,d][7]
annulene-5,11-dioxime and electrospray ZnO.
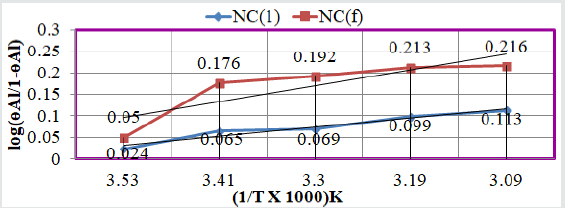
Corrosion rate of Al with log(ɵAl/1-ɵAl): The values of
log(ɵAl/1-ɵAl) for tetrahydro-dibenzo[a,d][7]annulene-5,11-
dioxime and ZnO verse 1/T plotted in Figure 3 and their values
were written in (Table1). The results of (Table 1) and (Figure 3)
were shown that the values of log(ɵAl/1-ɵAl) versus 1/T decreased
after coating.
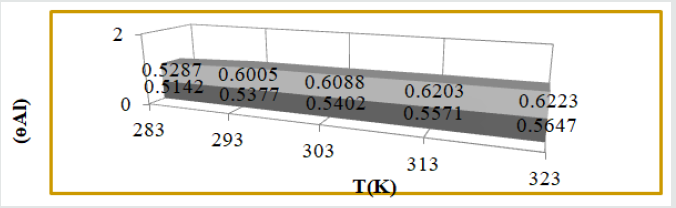
Surface coverage area developed by tetrahydro-dibenzo[a,d][7]
annulene-5,11-dioxime and ZnO: The covered area was calculated
by equation, ɵAl = (KAlo-KAl/KAl) and their values were given
in (Table 1). The graph of Figure 4 plotted between ɵAl(surface
coverage area) versus T(temperature) for tetrahydro-dibenzo[a,d]
[7]annulene-5,11-dioxime and ZnO. It observed that nanocoating
and electrospray compounds increased surface coverage areas
in SO2 environment. Nanocoating and electrospray developed a
passive composite barrier that is suppressed the attack of SO2.
Table 1:Corrosion rate of Al with nanocoating of tetrahydro-dibenzo [a,d] [7]annulene-5,11-dioxime [Ct] and ZnO in SO2 environment.
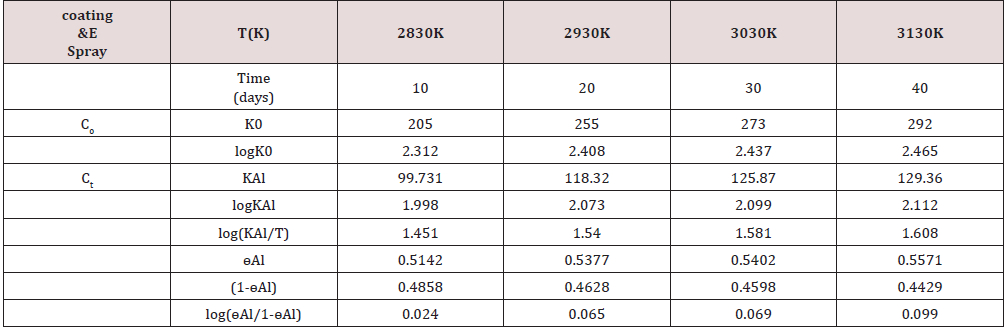
( Co = Uncoated, Ct = tetrahydro-dibenzo [a,d] [7] annulene-5,11-dioxime)
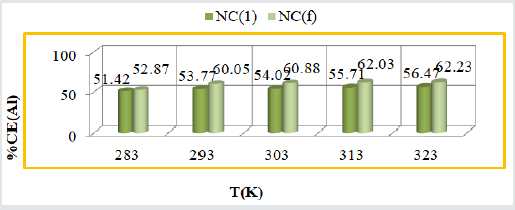
Percentage coating efficiency of tetrahydro-dibenzo [a,d] [7] annulene-5,11-dioxime and ZnO: The percentage coating efficiencies were determined by equation, % CE(Al) = (KAlo-KAl/KAl) X 100 and their results were written in (Table 1). (Figure 5) indicated the graph between % CE (Al) (percentage coating efficiency) versus T (temperature) which indicated that electrospray compound increased more coating efficiency with respected of nanocoating compound.
Activation energy of coated tetrahydro-dibenzo [a,d] [7] annulene-5,11-dioxime and ZnO:
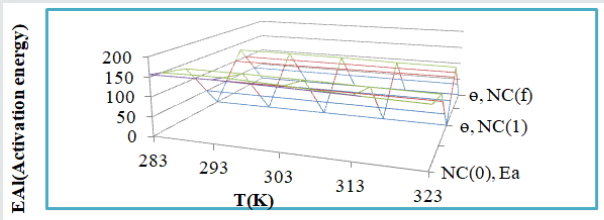
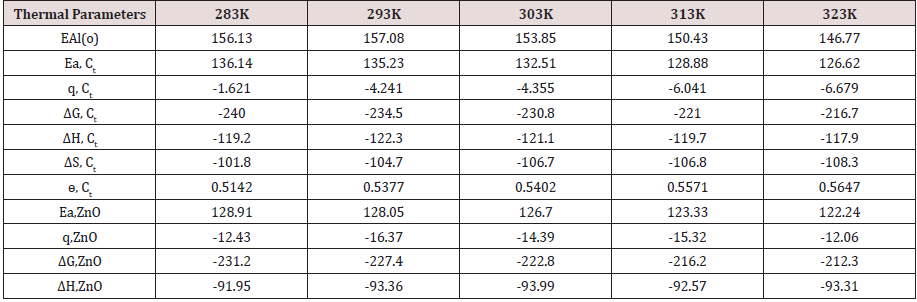
The activation energies of Al without coating and coating with tetrahydro-dibenzo[a,d][7]annulene-5,11-dioxime and ZnO were obtained by Arrhenius equation d /dt (logKAl) = EAl/ R T2 (where T is temperature in Kelvin, R is universal gas constant and EAl is the activation energy of the reaction) and (Figure 2). Its values were recorded in (Table 2). (Figure 6) plotted against EAl (activation energy) versus T (temperature) and ɵAl(surface coverage area). Activation energy reduced as temperature enhanced and surface coverage area occupied by nanocoaing and electrospray compounds increased. This trend was clearly observed in (Figure 6) and their values indicated that nanocoating and electrospray were attached with Al by chemical bonding. The values of activation energy for tetrahydro-dibenzo[a,d][7]annulene-5,11-dioxime and ZnO were mentioned in (Table 2) which indicated these compounds have less energy so they adhered with base metal by chemical bonding.
Heat of adsorption of tetrahydro-dibenzo [a,d] [7] annulene-5,11-dioxime and ZnO:
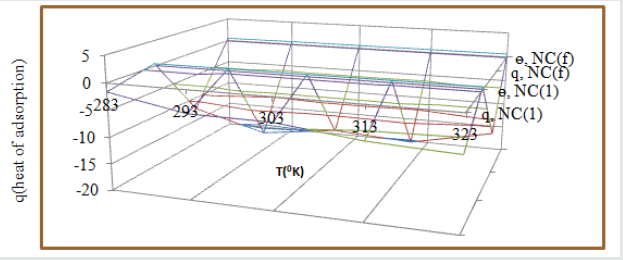
The heat of adsorption coating and electrospray compounds were calculated by equation Langmuir equation, log (θAl/ 1-θAl) = log (A .C) - (qAl / 2.303 R T) (where T is temperature in Kelvin and qAl heat of adsorption) and (Figure 3). The values of heat of adsorption of both compounds were recorded in (Table2) and values were found to be with negative. These results confirmed that both compounds were adsorbed on the surface of Al by physical bonding. Heat of adsorption increased at lower to higher temperatures and surface coverage area also enhanced by nanocoating and electrospray compounds as shown in (Figure 7).
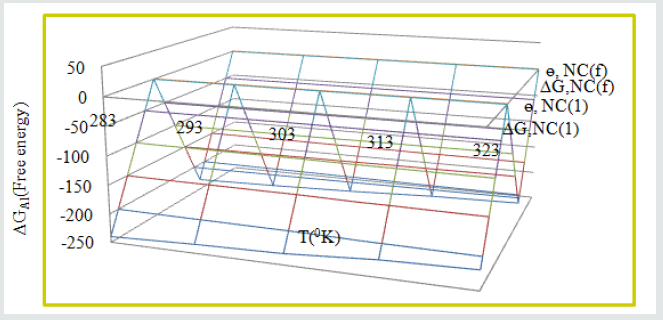
Free energy of tetrahydro-dibenzo [a,d] [7] annulene- 5,11-dioxime and ZnO:
The values of free energy of coating and electrospray compounds were obtained by equation, ΔGAl = -2.303RT log (33.3KAl) (where R is universal gas constant, T be temperature and K corrosion rate) and their values were mentioned in (Table 2). These values were indicated nanocaoting and electrospray compounds formed surface film on base material by chemical bonding. (Figure 8) exhibited that free energy reduced at higher temperature and surface coverage area increased with both compounds.
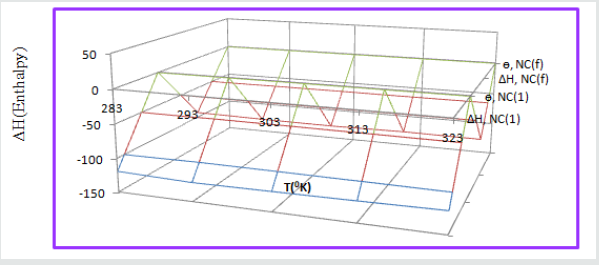
Enthalpy of tetrahydro-dibenzo [a,d] [7] annulene-5,11-dioxime and ZnO:
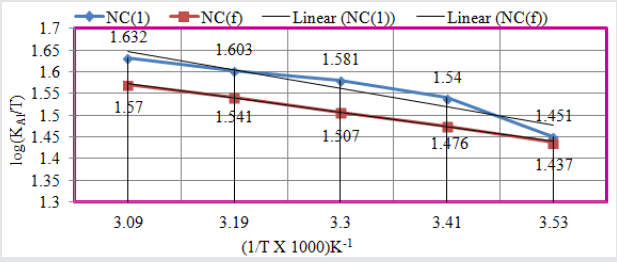
The values of enthalpy of both compounds were calculated by transition equation, K = R T / N h log (ΔSAl# / R) X log (-ΔHAl #/ R T) (where N is Avogadro’s constant, h is Planck’s constant, ΔSAl# is the change of entropy activation and ΔHAl # is the change of enthalpy activation) and plot of log(KAl/T) verse 1/T in (Figure 9) and their values were written in (Table 2). The enthalpy of both compounds indicated that they were adsorbed on the surface of Al by chemical bonding. It was observed that enthalpy values decreased when temperatures enhanced and surface coverage area increased as shown in (Figure 10). The enthalpy results were indicated that exothermic process occurred during coating.
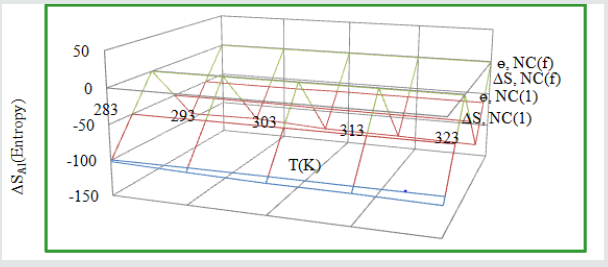
Entropy of tetrahydro-dibenzo [a,d] [7] annulene-5,11- dioxime and ZnO:
The values of entropy of both compound mentioned in (Table2). It indicates that nanocoating is an exothermic process. The nanocoating and electrospray compounds were accommodated by chemical bonding. (Figure11) depicted that entropy of both decreased when temperatures were increased and they enhanced surface coverage area. Nanocoating tetrahydro-dibenzo[a,d][7] annulene-5,11-dioxime and ZnO electrospray were arranged on the surface of Al in order manner. Entropy values of both compounds were shown that nanocoating is an exothermic process. The thin film barrier developed by tetrahydro-dibenzo[a,d][7]annulene-5,11- dioxime and ZnO to mitigate corrosion of Al in SO2 environment.
Potentiostatic polarization of tetrahydro-dibenzo [a,d] [7] annulene-5,11-dioxime and ZnO:
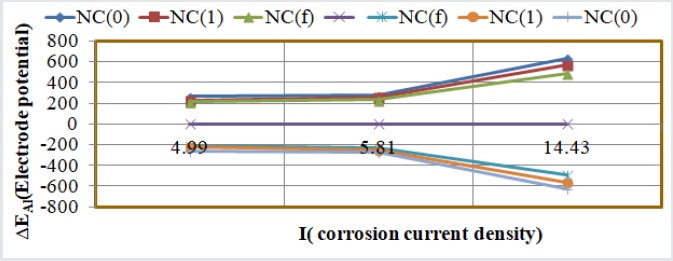
The results of electrode potential, corrosion current density, anodic and cathodic polarization were obtained by equation, ΔE/ΔI = βa βc / 2.303 Icorr (βa + βc) (where ΔE/ΔI is the slope with linear polarization resistance (Rp), βa and βc are anodic and cathodic Tafel slope respectively and I is the corrosion current density in mA/cm2) and Tafel plot between electrode potential (ΔE) versus current density (I) and their values were written in (Table 3). The results of (Table 3) and Tafel plots shown in (Figure 12) between corrosion potential and corrosion current density informed that Al produced higher corrosion potential but its values were reduced with tetrahydro-dibenzo[a,d][7]annulene-5,11-dioxime and ZnO. It was also observed that Al developed more corrosion current density whereas nanocoating and electrospray compounds reduced their values. The anodic polarization current increased with polymeric-coated Al but cathodic polarization current enhanced by nanocoating and electrospray compounds.
Table 3:potentiostatic polarization of tetrahydro-dibenzo[a,d][7]annulene-5,11-dioxime and ZnO nanocoating on Al.
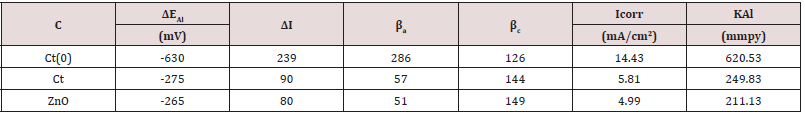
Putting the values of corrosion current density of polymericcoated Al, nanocoating tetrahydro-dibenzo[a,d][7]annulene-5,11- dioxime and ZnO electrospray in equation, C. R (mmpy) = 0.1288 I (mA /cm2) × Eq .Wt (g) / ρ (g/cm3) (where I is the corrosion current density ρ is specimen density and Eq.Wt is specimen equivalent weight) produced corrosion rate of material in each case and their values were given in (Table 3). In these results it was noticed that corrosion rate enhanced with polymeric-coated Al but nanocoating and filler compounds reduced the same. Gravimetric results were produced by Al,tetrahydro-dibenzo[a,d][7]annulene-5,11-dioxime and ZnO were confirmed by the result of potentiostat. The results of potentiostat obtained for nanocoating of tetrahydro-dibenzo[a,d] [7]annulene-5,11-dioxime and ZnO electrospray, it could shown that they produced high current density and neutralized the attack of SO2. The surface coverage area and percentage coating efficiency tetrahydrodibenzo[a,d][7]annulene-5,11-dioxime and ZnO were confirmed that electrospray enhanced more these values and provide coating barrier thermal, chemical, physical and mechanical stability.
Mechanism of tetrahydro-dibenzo [a,d] [7]annulene- 5,11-dioxime and ZnO
It is electron rich compound because it contains di-oxime functional groups and benzene ring. Coating of this compound, it is bonded with Al metal through chemical bonding. After coating of tetrahydro-dibenzo[a,d][7]annulene-5,11-dioxime lots of porosities develop on base metal. These porosities block with electrospraying of ZnO thus a composite barrier is produced. The composite barrier of tetrahydro-dibenzo[a,d][7]annulene-5,11-dioxime and ZnO stop diffusion of SO2 and base metal can be protected.
Conclusion
The nanocoating of tetrahydro-dibenzo[a,d][7]annulene-5,11- dioxime and ZnO is an exothermic process. These compounds adsorbed on Al metal through chemisorptions process that is confirmed by activation energy, heat of adsorption, free energy, enthalpy and entropy. The composite barrier created by tetrahydrodibenzo[ a,d][7]annulene-5,11-dioxime and ZnO which produced strong chemical bonding. Both compounds reduced corrosion rate and enhanced surface coverage area and coating efficiency. The composite barrier developed by tetrahydro-dibenzo[a,d][7] annulene-5,11-dioxime and ZnO work as repeller for SO2 and checked its diffusion process.
Acknowledgement
Author is thankful for UGC, New Delhi to provide finical support. I give my regard research scholars and laboratory staffs for their data collection.
References
- Aboia OK, James AO (2010) The effects of Aloe Vera extract on corrosion and kineties of corrosion process of zinc in HCl solution. Corrosion Science 52(2): 661-664.
- Eddy NO, Odoemelam S A (2009) Inhibition of corrosion of mild steel in acidic medium using ethanol extract of Aloe Vera. Pigment and Resin Technology 32(2): 111-115.
- Bouyaner A, Hammout B (2006) Pennyroyal oil from Menthapulegium as corrosion inhibitor for steel in M HCl. Materials Letters 60(23): 2840-2843.
- de Souza FS, Spinelli A (2009) Caffeic acid as a green corrosion inhibitor in mild steel, Corrosion Science 51(3): 642-649.
- Uwah IE, Okafor PC, Ebiekpe E (2013) Inhibitive action of ethanol extracts from Nauclea latifolic on the corrosion of mild steel in H2SO4 solution and their adsorption characteristics”, Arbian Journal of chemistry 6(3): 285-293.
- Lebrine M, Robert F, Roos C (2010) Inhibition effects of alkaloids extract from Annona Squamosa plant on the corrosion of C38 Steel in normal hydrochloric acid medium”, International Journal of Electrochemical Science 5(11): 1678-1712.
- Okafor PC, Ebenso EE (2010) Azadira chat indica extracts as corrosion inhibitor for mild steel in acidic medium”, International Journal of Electrochemical Science 5(7): 978-993.
- Oguzie E E (2008) Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corrosion Science 50(11): 2993-2998.
- Sethuram MG, Raja PB (2005) Corrosion inhibition of mild steel by Datura metal in acidic medium. Pigment and Resin Technology 34(6): 347-352.
- Satapathy A K, Gunaskaran G (2009) Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corrosion Science 51(12): 2848-2856.
- Badiea AM Mohana KN (2009) Corrosion mechanism of low carbon steel in industrial water and adsorption thermodynamics in the presence of some plant extract. Journal of Materials Engineering and Performance 18(9): 1264-1271.
- Rajenderan S, Jeyasundari J, Usha P (2009) Corrosion behavior of aluminium in the presence of an aqueous extract of hibiscus rosansensis. Portugaliae Electrochemica Acta 27(2): 153-164.
- Kumar S, Arora S, manish sharma, paresh arora, suraj prakash mathu, et al. (2009) Synergistic effect of Calo tropis plant in basic solution. Journal of the Chilean Chemical Society 54(1): 83-88.
- Sivaraju M, Kannan K (2010) Eco-friendly inhibitor (Tributes terres trist) for mild steel corrosion in 1NH phosphoric acid. Asian Journal of Chemistry 22(1): 233-244.
- Okafor PC, Uwash IE, Okpo Ekerenam, Ekpe UJ (2009) Combretum bracteosum extracts as eco-friendly corrosion inhibitor for mild steel in acidic medium. Pigment and Resin Technology 38(4): 236-241.
- Qurasishi MA, Singh A, Singh VK (2009) Green approach to corrosion inhibitor of mild steel in hydrochloric acid and sulphuric acid solution by the extract of Murraya Koenigii leaves. Materials Chemistry and Physics 122(1): 114-122.
- Singh RK, Noor A (2019) Study the Corrosion & Corrosion Protection of Brass Sculpture by Atmospheric Pollutants in Winter Season. Modern Approach on Material Science 1(3): 54-62.
- Singh RK, Thakur K, Manjay (2017) Mild Steel Corrosion Control by Nanocoating and Filler Compounds in Hostile Environments. J of J Material Science 4(3): 1-12.
- Singh RK (2017) Corrosion Protection of Materials by Nanocoating of Decahydrobenzo[8]annulene-5,10-diphenyhydrazone and AlN filler. International Journal of Bio-Innovation 6(5): 828-846.
- Singh RK (2017) Corrosion Protection Polybutadiene-coated Mild steel in Marine Water by Nanocoating and filler Compounds. Research & Developments in Materials Science 1(1): 1-7.
- Singh RK, Singh MK, Noor A (2019) Organic compound 5-(4-methoxybenzyl)-N-Phenyl-1,3,4-thiadiazol-2-amine and NiS filler used to mitigation the corrosion of mild steel in marine atmosphere”, International Journal of Metallurgy and Alloys 5(2): 56-64.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...