Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Research Article(ISSN: 2641-1725) 
Differential Molecular Investigation Assay of Tumor Nicrosis Factor –Alpha (TNF-α), Interleukin-1 Beta (IL-1β), Interleukin-6 (IL-6), and Interleukin 10 (IL-10) among Children Potentially Diagnosed of Falciparum-Malaria of Niger Delta Extract Volume 4 - Issue 2
Azuonwu O*, Wokem GN and Dimkpa FB
- Department of Medical Laboratory Science, Rivers State University, Nigeria
Received: December 02, 2019; Published: December 17, 2019
*Corresponding author: Azuonwu O, Department of Medical Laboratory Science, Rivers State University, Port Harcourt, Nigeria
DOI: 10.32474/LOJMS.2019.04.000181
Abstract
This cross-sectional study determined absolute parasitaemia and evaluated some inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and interleukin-10 (IL-10) concentrations in children infected with Plasmodium falciparum parasites in Port Harcourt, Rivers State, Nigeria. A total of 160 blood samples were randomly and aseptically collected through convenient sampling technique research design and were analysed respectively. The plasma obtained from the blood samples after centrifugation at 1500 rpm for 5 minutes were subjected to cytokine evaluation analysis using, commercially purchased standard Enzyme Linked Immunosorbent Assay kit (ELISA) in line with manufactures instructions- (ELISA - Elabscience Biotechnology Inc, USA). Malaria diagnosis and blood parameter assays were carried out using standard diagnostic techniques of haematological and parasitological benchmark standard respectively. However, result from the study showed that children within the age group 9-11 and 6-8 years had the highest and lowest mean parasitaemia levels of 4813.09±1180.05 parasites/µl and 2324.62±546.63 parasites/µl respectively (P=0.048). The mean concentration levels of IL-6, and IL-10 (365.30±40.31 pg/ml, and 318.57±39.29 pg/ml respectively) were significantly higher in children with severe parasitaemia (P=0.0014, and 0.0347 respectively).The mean cytokine levels of TNF-α, IL-β, IL-6,and IL-10, were significantly elevated in children with P. falciparum infection when compared to their respective healthy matched controls (P<0.0001). Correlations between plasma levels of IL-6 and P. falciparum parasite density in children showed a relatively strong positive relationship (P<0.0001). Inflammatory cytokines are involved in immunopathogenesis and immunoregulation of Plasmodium falciparum infection. Nonetheless, cytokines as found in this study could be used as a promising evidence based prognostic and diagnostic biomarkers for falciparum malaria progression or regression in our health facilities in the region.
Keywords: Inflammatory cytokines; Plasmodium falciparum; Parasitaemia; Malaria; Children; Niger Delta; Biomarker
Introduction
Malaria is a life-threatening disease caused by obligate intracellular Plasmodium parasites such as Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, Plasmodium vivax, and Plasmodium knowlesi [1] Sporozoites, the infective stage of malaria parasites are notably transmitted via the bites of infected female Anopheles mosquitoes. Children who reside in poverty endemic areas in Africa are more susceptible to Plasmodium falciparum infection. Nonetheless, in 2016, about 285,000 African children less than 5 years of age died of malaria before their fifth birthdays [2] Also, about 219 million clinical cases of malaria were reported in 87 countries globally in 2017, with an estimated 435,000 deaths [1,3]. In sub-Saharan Africa, Plasmodium falciparum is the most prevalent and deadly malaria parasite which accounted for 99.7% cases of malaria-hospitalization in 2017 [4]. However, Nigeria is one of the malaria burdened countries in West Africa [4] Infants and children under 5 years of age, naïve adults, and pregnant women are critically very much vulnerable to Plasmodium falciparum malaria in endemic settings [1,5,6]. It is strongly believed that Malaria infection is severe and fatal in children leading to 1 in 5 cases of childhood death in sub-Saharan Africa, [7] possibly due to their relative poor or partial immunity to the infection [2, 8].
Nevertheless, given the huge public health implications posed by malaria burden in Africa, especially in the remote communities of Sub- Sahara Africa, with no visible functional health facilities, thus, there is an urgent need to explore complementary immuno-diagnostic cum preventive strategies to curbing the menace of malaria, especially among children in sub-Saharan Africa. Nevertheless, evidence based research has shown that many attempts to develop effective malaria vaccine yielded unsatisfactory outcomes, probably due to the complexity of Plasmodium falciparum parasite [9]. However, recently the first documented malaria vaccine, RTS, S/ASO1 (RTS, S), against Plasmodium falciparum was introduced in three pilot African countries, such as Malawi, Ghana, and Kenya respectively. This first malaria vaccine does not confer absolute immunity but offers partial resistance against falciparum malaria in young African children [1]. Hence, the urgent need to developing effective vaccine as a strategic intervention for global prevention of malaria in children remains sacrosanct and a task that must be done to save the lives of the weak and most vulnerable subjects in our communities across the malaria endemic regions. The complex pathogenesis of P. falciparum malaria appears to involve dysregulation of the immune system, therefore, a better knowledge of mechanisms of protective immunity and immunopathology of falciparum malaria would provide significant clues on how to explore the immune system to potentially actualize the goals of developing an improve and robust vaccines against malaria parasiteamia.
Nevertheless, cytokines are glycoproteins secreted by variety of cells that regulate the host immune response to foreign antigens such as infectious agents, and to other stimuli such as inflammation, and trauma. Cytokines act as immune modulating agents, thus assist in the regulation and development of the effector phases of both innate and specific immune responses through the activities of neutrophil-rich inflammatory responses, and activated T lymphocytes respectively [10,11]. Some inflammatory cytokines are implicated in the immunopathogenesis of falciparum malaria. Several studies have shown that glycosylphosphatidylinositols (GPIs) from Plasmodium falciparum has toxigenic property which can stimulate tumor necrosis factor- alpha (TNF-α), interleukin -1 (IL-1), interleukin-6 (IL-6), and interferon gamma (IFN-γ) in macrophages [12,13]. Study has also, shown that the naturally acquired anti-glycosylphosphatidylinositol antibodies in the serum of malaria patients neutralize GPIs and thereby, may provide immune-resistance against clinical symptoms of severe malaria [13]. Furthermore, similar study suggested that changes in concentrations of some inflammatory cytokines and chemokines in plasmodiasis may prove useful in evaluating either the progression or the retrogression of uncomplicated to severe falciparum malaria [14]. It has been reported that excessive production of proinflammatory cytokine, tumor necrosis factor (TNF), can cause pathology while early production of proinflammatory T helper type 1 (Th1) cytokines, tumor necrosis factor (TNF), interleukin -12 (IL-12), and possibly interferon- gamma (IFN-γ) may limit the progression from uncomplicated malaria to severe, and other life-threatening complications [14]. Several studies agree that Th1 responses are important for clearance of falciparum malaria. Hence, elevated levels of interleukin-6 (IL-6), and interleukin -10 (IL-10), were observed in serum of patients with severe P. falciparum malaria [14,15]. In many parts of Africa, interventions such as the use of long-lasting insecticidal nets (LLINs), and WHO-recommended chemotherapies have reduced the burden of malaria [16]. However, several studies incriminated such prevention strategies of enhancing the upsurge of drug-resistant Plasmodium falciparum, and insecticide-resistant female Anopheles mosquitoes [17,18,19]. This study measured absolute parasitaemia and variations in inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-10) among falciparum malaria-infected-children in Port Harcourt, Rivers State, Nigeria. Nonetheless, it is therefore strongly believed that data and robust recommendations that would be generated from this present study would provide enough leverage, background and massive workable practical impetus that would improve on prompt diagnosis, management and prevention of malaria parasiteamia among children in the region.
Materials and Methods
Study area
This cross-sectional study was conducted in Health facilities in Port Harcourt (Braithwaite Memorial Hospital and College of Health and Technology Health Services Clinics) between the months of July, 2017 to October, 2018. Nonetheless, Port Harcourt is the largest city in Rivers State, located at 40 45 IN 60 50 IE / 4.750 0N 6.8330 E of Nigeria within the oil-rich Niger Delta, South-South geopolitical zone of Nigeria. The climate of the area is characterized by rainy and dry seasons. The rainy season starts in May and last till October, with July and August as Months of peak rainfall. The dry season which starts from December to March are marked with periodic harmattan and very dusty. The vegetation of the study area reflects that of the mangrove rainforest zones. Anopheles gambiae complex is the main malaria vector in Nigeria while P. falciparum is the most prevalent malaria species [7,20,21].
Ethical statement
Ethical permission was obtained from the State Ministry of Health and Rivers State University Teaching Hospital, Port Harcourt ethical committee respectively. Also, informed consent was obtained from participants or their respective parents or guardians before sample collection process was activated.
Exclusion criteria
Children with mixed infection of malaria parasites and those with overt chronic infections were excluded. Also children whom their parents could not consent to be included in the study was out rightly removed from the study population, even as adults were removed from the study.
Inclusion criteria
Those who their parents consented were included in the study. Only children were recruited for the study, even as those with chronic illness were carefully excluded from the study.
Experimental
A total of 160 blood samples were selected for the study out of which, eighty (80) samples (n=80) were positive for P. falciparum parasites while another 80 samples were (n=80) negative for P. falciparum parasites from children, thus those negative samples were used as healthy matched controls settings for the study.
About 5mls of venous blood were collected aseptically into EDTA container, labelled with barcode and taken to laboratory for analysis. Thick and thin malaria blood films were made as described by Cheesbrough [22]. The slides were stained with 3% solution of Giemsa stain as described by World Health Organisation procedure protocol [23]. The presence of P. falciparum parasitaemia was determined by microscopic examination of thick and thin smears using x100 oil immersion objective field respectively.
Determination of absolute parasite density
The absolute parasite density of the children infected with P. falciparum parasites was determined and calculated as described by Agomo et al. [24]; the total leucocyte count determination for each subject was done using Sysmex XP-300 automated haematology analyzer according to the manufacturers’ instructions.
Determination of degree of Parasitaemia
The degree of parasitaemia was graded as described by Adesina et al. [25] Parasitaemia with absolute parasite density of less than 1000 per microlitre of blood (<1000 parasites/µl) was graded as low parasitaemia; parasitaemia with absolute parasite density of greater than 1000 per microlitre of blood but less than 10,000 per microlitre of blood (>1000 to 9,999 parasites/µl) was graded as moderate parasitaemia while parasitaemia with absolute parasite density of greater than 10,000 per microlitre of blood (>10,000 parasites/µl) was graded as high parasitaemia.
Determination of cytokine concentration in plasma
The in vitro quantitative determination of cytokine concentrations in plasma such as human IL-6, IL-1β,TNF-α, and IL-10 were measured using the sandwich enzyme-linked immunosorbent assays (ELISAs) technique with Human IL-6, IL-1β,TNF-α, and IL-10 Enzyme Linked Immunosorbent Assay Kits according the manufacturers’ instructions (Catalog Numbers: E-EL-H0102; E-EL-H0149; E-EL-H0109; and E-EL-H0103 Elabscience® , USA). After securing the desired number of pre-coated micro ELISA plate wells in the micro-well holder, 100 µl of standards of various concentrations, samples and controls were dispensed into appropriate wells. The micro-plate wells were covered with the sealer provided in the kit and incubated for 90 minutes at temperature of 37 degree centigrade (37 °C). This was followed by removal of the liquid out of each well into the sink, then the plate was placed face down on absorbent paper with some force to remove any remaining liquid from the wells. Immediately 100µl of biotinylated detection antibody was added to each well, covered with the plate sealer, gently mixed up and incubated for 60 minutes at 37 °C. After incubation, the solution was aspirated from each well, 350µl of wash buffer was added to each well, soaked for one to two minutes and was further aspirated from each well. Aspiration and washing was repeated thrice, while the remaining liquid from the wells were removed by striking the wells sharply with the plate face down on absorbent paper at the end of washing. Then, 100µl of HRP conjugate working solution was added into each well. It was covered with the plate sealer and gently mixed and incubated for 30 minutes at 37 °C. Then, the plate was aspirated and washed thoroughly for five times. Ninety microlitres (90µl) of substrate reagent was added to each well, was covered with new plate sealer, and incubated for 15 minutes at 37 °C in the dark.
Following incubation, the reaction was stopped by adding 50µl of stop solution to each well. A qualitative result was observed with respect to sample colour changed from blue to yellow completely. The optical density value of the coloured solution in each well at once, was determined using a micro-plate reader (Rayto Microplate Reader RT-2100C, China) at 450nm wavelength. Analysis was performed in duplicates. Cytokines concentrations (pg/µL) were determined using the standard curve and multiplied by the dilution factor. The detection range of cytokine assayed was 7.81-500 pg/µL for IL-1β, IL-6, TNF-α, and IL-10.
Statistical Analysis
The data generated from this study were analysed using tables and statistical tools. Statistical analysis was carried out on the various groups of data generated from this study with the aid of Microsoft Excel version 2007 and statistical package for social sciences (SPSS) version 23, using analysis of variance (ANOVA), Pearson correlation tests, and student t-tests. Results were presented in graphs and tables. The level of statistical significance was set as P < 0.05.
Results
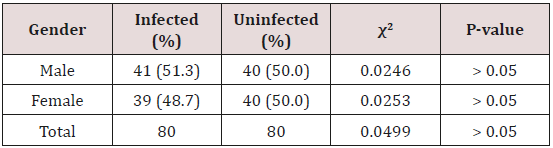
Table 1 shows the gender-related distribution of children infected with P. falciparum parasites. The male had higher prevalence, 51.3%, compared with the female children who had a prevalence of 48.7%, however, the difference between them was not statistically significant (χ2=0.0499; P > 0.05).
Figure 1: Positive Correlation between Parasite Density and TNF-α Plasma Levels in Children Infected with P. falciparum (r=0.16; P=0.159).
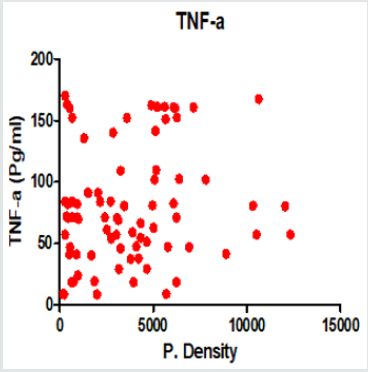
Figure 2: Positive Correlation between Parasite Density and IL-1β Plasma Levels in Children Infected with P. falciparum (r=0.26; P=0.021).
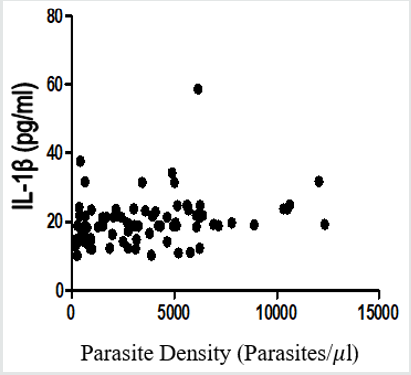
Table 2 shows the various mean parasitaemia of P. falciparum malaria-infected children according to age. Those within the age group of 9-11 years had the highest mean ± SE parasite density of 4813.09±1180.05 parasites/µl, while those within the age group of 6-8 years had the lowest parasite density of 2324.62±546.63 parasites/µl. Comparisons of the mean parasitaemia showed a statistical significant difference among the different age group of children (P=0.048).
Table 3 shows comparisons of the mean concentrations of cytokines and degree of parasitaemia among P. falciparum malaria-infected children studied. Tumor necrosis factor alpha (TNF-α) had a mean concentration of 88.64 ± 20.42 pg/ml in severe parasitaemia, 81.79 ± 6.36 pg/ml in moderate parasitamia, and 72.77±10.55 pg/ml in mild parasitaemia. Comparisons of the mean concentrations of TNF-α in various degree of parasitaemia was not statistically significant (P=0.6857). The mean concentration of IL-Iβ was higher in severe parasitaemia (24.67±2.00 pg/ml) compared with moderate parasitaemia (20.39±1.00 pg/ml) and mild parasitaemia (18.45 ± 1.38 pg/ml). However, comparisons of their means showed no statistically significant difference (P=0.1816). Interleukin-6 (IL-6) had a higher mean concentration in severe parasitaemia (365.30 ± 40.31 pg/ml), 172.48 ± 19.61pg/ml in moderate parasitaemia and 111.44 ± 26.96 pg/ml in mild parasitaemia. Comparisons of their mean concentrations showed a statistical significant difference (P=0.0014). Interleukin-10 (IL-10) had mean concentrations of 318.57±39.29 pg/ml in severe parasitaemia, 230.26 ±16.08 pg/ml in moderate parasitaemia and 178.51±23.85 pg/ml in mild parasitamia. Comparisons of the mean concentrations showed a statistically significant difference (P=0.0347).
Figure 3: Positive Correlation between Parasite Density and IL-6 Plasma Levels in Children Infected with P. falciparum (r=0.43; P<0.0001).
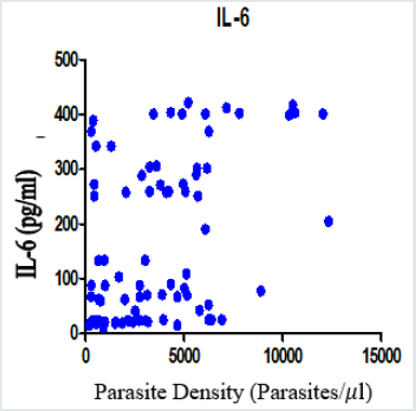
Table 4: Comparisons of Mean SEM Cytokines Levels of P. falciparum Malaria Infected Children and Un-infected Children.

Table 5. Comparisons of Mean SEM Cytokines Levels of P. falciparum Malaria Infected Children and Un-infected Children.

Figure 4: Positive Correlation between Parasite Density and IL-10 Plasma Levels in Children Infected with P. falciparum (r=0.28; P=0.013).
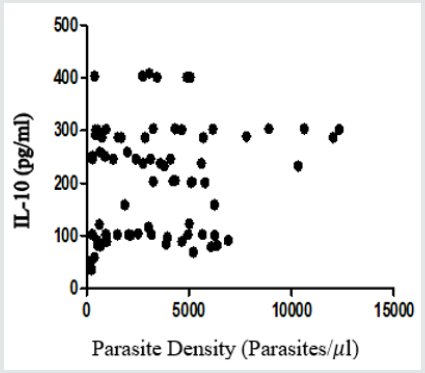
Table 4 shows the mean concentrations of cytokines such as TNF-α, IL-Iβ, IL-6 and IL-10 evaluated in P. falciparum malaria-infected children by treatment. The concentration of TNF-α was 30.46 ± 1.74 pg/ml in uninfected children and 79.74 ±5.23 pg/ml in infected children. Comparisons of their mean concentrations showed a statistically significant difference (P<0.0001). Interleukin-1 beta (IL-Iβ) had a mean concentration of 6.46±0.56 pg/ml in uninfected children and 20.12±0.78 pg/ml in infected children (P<0.0001). Interleukin-6 (IL-6) had a mean concentration of 25.69± 2.54 pg/ml in uninfected children and 167.80 ± 16.37 pg/ml in infected children (P< 0.0001). The mean concentrations of IL-10 was 62.64 ± 6.47 pg/ml and 218.90 ± 12.82 pg/ml in uninfected and infected children respectively, and the comparisons of the mean concentrations of IL-10 was statistically significant (P < 0.0001).
Figure 1 to Figure 4 show Pearson correlation between malaria parasite densities and TNF-α, IL-Iβ, IL-6, IL-10 plasma levels in children infected with P. falciparum parasite. There was a positive linear relationship between malaria parasite densities and TNF-α, IL-Iβ, IL-6, IL-10, as shown in Figure 4.5, 4.6, 4.7, and 4.8 (r = 0.16, 0.26, 0.43, 0.28 respectively). The positive correlation between malaria parasite density and TNF-α plasma levels in children infected with P. falciparum parasite was not statistically significant (r = 0.16, P=0.159). However, the positive relationships between malaria parasite densities and IL-1β, IL-6, and IL-10 plasma levels in children infected with P. falciparum parasite were statistically significant (P=0.021; P < 0.0001; and P= 0.013 respectively).
Discussion
Plasmodium falciparum is the most deadly and prevalent
malaria parasite in Nigeria. This study determines absolute
parasitaemia and evaluates variation in levels of some inflammatory
cytokines released during infection with P. falciparum parasites
in children. Malaria infection caused by Plasmodium falciparum
induces immune responses in children. Children are among the
high risk group to malaria due to none or weak immune resistance
capacity at that level.
Nmadu et al. [26] reported an overall prevalence of 62.5% of
Plasmodium falciparum parasite among children aged 2 to 15
years who visited Gwarinpa general Hospital Life-camp, Abuja. In
similar study conducted in Yola, Adamawa State, Northern Nigeria,
a total prevalence of 50.6% of malaria parasites was reported
among children who attended specialist hospital in Yola [27].
Also, in Anambra, South East Nigeria, Nwaorgu and Orajaka [28]
reported a prevalence of 51.8% of Plasmodium falciparum among
pupils recruited from both primary and nursery schools. These
previous findings agree with the overall prevalence of Plasmodium
falciparum parasite reported in this study. However, the finding in
this study contrast with the prevalence of 94.6% of Plasmodium
falciparum reported in another related hospital-based research
conducted in Anambra State, South East Nigeria [29]. The variation
in rates of Plasmodium faciparum parasitaemia as reported by
various researchers might suggest different techniques of malaria
parasite determination and various levels of malaria endemicity in
the affected geographical settings where the studies were carried
out. This study was carried out during the wet season, a period
that favours the breeding of Anopheles gambiae complex, vectors
of malaria parasites. This study observes a steep increase in agerelated
prevalence of Plasmodium falciparum among children
with decrease in ages. This finding corroborates with the research
work conducted in Abuja among children of similar age range. This observation supports the position that in malaria endemic
areas, children are more at risk to malaria infection which might
be due to undeveloped or weak immunity that is influenced by age.
Secondly, in those endemic locations especially in the hinterlands,
children are seen playing outside in the evening hours, without
much covering of their bodies with cloths, thereby promoting
mosquitoes bite access, even as some of them are used as hawkers
or street traders which also potentially exposes them unabatedly to
increasing attacks of mosquitoes carrying malaria parasite
Nonetheless, Table 2 shows comparisons of mean parasitaemia
of P. falciparum malaria-infected children in relation to their various
ages. It was observed that children within the age range of 9-11
years had the highest mean parasitaemia level, 4813.09 parasites
/μl, followed by those within the age range of 3-5 years, which had
a mean parasitaemia level of 4213.95 parasites /μl. However, the
mean parasitaemia levels in children infected with uncomplicated
malaria reported by Nmorsi et al. [30] were slightly lower, 3158.8
parasites /μl compared with that reported in this study. The
observed differences might be cause by different techniques of
estimating density of parasitaemia and different levels of exposure
to P. falciparum. Although the differences in the mean parasitaemia
levels of children infected with falciparum malaria in this study was
statistically significant (P< 0.05).
This study is consistent with earlier studies which demonstrated
that P. falciparum infection triggers immune responses in children,
[14,15] leading to production of inflammatory cytokines. Several
studies have shown that pathogen-associated molecular patterns
(PAMPs) of P. falciparum, glycosylphosphatidylinositols (GPls)
activates signal-tranducing proteins called toll-like receptors (TLR2
and TLR4) of innate immune cells to induce proinflammatory
cytokines such as TNF-α, IL-Iβ, IL-6, which can contribute to
malaria parasite pathogenesis [31,32]. Haemozoin, parasites
membrane-derived particles, and uric acids have been identified as
potent mediators of P. falciparum-induced inflammatory response
that also contributes in the secretion of cytokines in malaria [14].
Findings in this study Table 4 show significant increase in
the concentrations of TNF-α, IL-Iβ, IL-6 and IL-10 in P. falciparum
malaria-infected children compared with their uninfected
counterparts. Plasmodium falciparum infection has long been
associated with high circulating levels of inflammatory cytokines
such as tumor necrosis factor alpha (TNF-α), IL-1β, and IL-6.
Furthermore, previous studies have demonstrated a link between
TNF-α, IL-6, IL-10 and the severity of the disease in human malaria
[33,34]. Anti-inflammatory cytokine, IL-10 has been incriminated
as an immunoregulator during Plasmodium falciparum Infection,
thereby neutralizing the effect of the other pro- inflammatory
cytokine, TNF-α, IL-16, produced by T helper 1 (Th1) cells Langhorne
et al. [35]. Interestingly, IL-10 is produced by monocytes, Th2 cells,
and B cells, and it inhibits inflammatory cytokines secreted by Th1
and CD8+ cells. Interleukin-10(IL-10) also induces the proliferation
of B cells, and synthesis of immunoglobulin, necessary for the
development and maturation of antibodies against malaria [8].
This study showed significant increase in levels of proinflammatory
cytokine, IL-6, and with anti-inflammatory cytokine,
lL-10, in children with mild, moderate and severe falciparum malaria
against their various matched respective healthy controls (Table 3)
(P< 0.05). Similarly, this agrees with the work of Jackobsen et al. [36]
which reported that excessive secretion of inflammatory cytokines
such as IL-6 in children infected with P. falciparum malaria depict
severity of malaria. This portrays the ability of human immune cells
to effectively mount strong immunity against P. falciparum antigens
in the study subjects.
Furthermore, mean concentration levels of proinflammatory
cytokines, TNF-α, IL- lβ, IL-6, and anti-inflammatory cytokine, IL-
10 were significantly elevated in falciparum malaria in infected
children and their respective healthy controls in this study
(P<0.0001). Nmorsi et al. [30] reported significantly higher levels
of proinflammatory cytokines among children with uncomplicated
and complicated malaria in Benin City, Edo, Nigeria. Several
reports have it that these inflammatory cytokines contribute in
preventing the replication of asexual stages of malaria antigens. In
this study, it was observed that increased levels of cytokines such
as TNF-α, IL-1β, IL-6, and IL-10 represent a normal level immune
response to P. falciparum infection. Although, excessive production
of inflammatory cytokines like IL-6 and IL-10 may predispose to
severe malaria and a fatal outcome. Anti-inflammatory cytokine,
IL-10, must be adequately sorted in P. falciparum cytokines to
inhibit the impact of pro-inflammatory cytokines (TNF -α, IL-1 β,
IL-6). Cytokine imbalance has been involved in the development of
complications observed among children with P. falciparum malaria
[8]. It has been reported that malaria tends to be more severe and
fatal in children than in adults, presumably because of undeveloped
immune system [8].
Conclusion
This study has demonstrated that pro-inflammatory cytokines such as TNF-α, IL-Iβ, IL-6 and anti-inflammatory cytokine, IL-10 are useful parameters for the prognosis and diagnosis of falciparummalaria infection in children. Also, the significantly elevated levels of IL-1β, IL-6, and IL-10 among children infected with P. falciparum parasites implicate these cytokines as the major mediators in the host responses to systemic, moderate, and severe parasitaemia of P. falciparum malaria in Southern Nigeria. The high levels of IL- 10 in this study suggest that IL-10 modulates pro-inflammatory cytokines which makes it significant in immune-regulation. This study demonstrates positive correlations between TNF-α, IL-1β, IL- 6, and IL-10 with P. falciparum parasitaemia.
Acknowledgement
We appreciate the children and their parents for their active participation in this study. We also acknowledge laboratory staff of Rivers State Teaching Hospital Port Harcourt and College of Health Science and Management Technology Health Centre, Port Harcourt, for their collaboration and assistance during sample collection and laboratory analysis.
Conflict of Interest
We know of no competing interests among authors.
References
- WHO (2019) Malaria. World Health Organization, Geneva, Switzerland.
- WHO (2018) Malaria: Malaria in children under five. World Health Organization, Geneva, Switzerland.
- WHO (2017) World malaria report. World Health Organization, Geneva, Switzerland.
- WHO (2018) Malaria. World Health Organization, Geneva, Switzerland.
- Doolan DL, Dobano C Baird J K (2009) Acquired immunity to malaria. Clin Microbiol Rev 22(1): 13-36.
- WHO (2010a) World malaria report. World Health Organization, Geneva, Switzerland.
- Wokem GN, Nnadi E, Azuonwu O, Okafor A (2018) Effect of malaria on selected liver function profiles of children in Port Harcourt, Rivers State, Nigeria. IJTDH, 31(4): 1-8.
- Tatfeng YM, Agbonlahor, DE (2010) Age-related cytokine profile in uncomplicated Plasmodium malaria infection. Colomb Med 41(4): 323-327.
- Kamuyu G, Tuju J, Kimathi R, Mwai K, Mburu J, et al. (2003) KILchip v1.0: A novel Plasmodium falciparum merozoite protein microarray to facilitate malaria vaccine candidate prioritization 9: 2866.
- Goldsby RA, Kindt TJ, Osborne BA, Kuby J. Immunology. W.H. Freeman and Company. New York, USA.
- Jayapal V (2007) Fundamental of medical immunology. Jaaypee Brothers Medical Publishers. New Delhi, India.
- Brattig NW, Kowalsky K, Liu X, burchard GD, Kamena F, et al. (2008) Plasmodium falciparum glycosylphosphatidylinositol toxin interacts with membrane of non-parasitized red blood cells: A putative mechanism contributing to malaria anaemia. Microbes and Infection 10(8): 885-891.
- Mbengue B, Niang B, Niang MS, Varela M L, Fall B, et al. (2016) Inflammatory cytokine and humoral responses to Plasmodium falciparum glycosylphosphatidylinositols correlates with malaria immunity and pathogenesis. ImmunInflamm Dis 4(1): 24-34.
- Ayimba E, Hegewald J, Segbena AY, Gantin RG, Lechner CJ, et al. (2011) Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum Clin Exp Immunol 166 (2): 218-226.
- Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, et al. (2004) Serum levels of proinflammatory cytokines interleukin- 1 beta (IL-β), IL- 6, IL -8, IL-10, tumor necrosis factor alpha, and IL-12 (p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 72 (10): 5630-5637.
- WHO (2013) World malaria report. Geneva, Switzerland.
- Noranata N, Durand A, Tall A, Marrama L, Spiegel A, et al. (2007) Rapid dissemination of Plasmodium falciparum drug resistance despite strictly controlled antimalarial use. PLoS ONE, 2 (1): e139.
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, et al. (2009) Artemisinin resistance in Plasmodium falciparum New Engl J Med, 361: 455-467.
- Tun KM, Imwong M, Iwin KM, Win AA, Hlaing TM, et al. (2015) Spread of artemisinin- resistant plasmodium falciparum in Myanman A cross- sectional survey of the K13 molecular marker. Lancet. J Infect Dis 15(4): 415-421.
- Okwa OO, Akinmolayan FI, Carter V, Hurd, H (2009) Transmission dynamics of malaria in fourselected ecological zones of Nigeria in the rainy season. Ann Afri Med 8(1): 1-9
- Olayemi IK, Ande AT, Ayanwale AV, Mohammed AZ, Bello IM, et al. (2011) Seasonal trends in epidemiological profiles of malaria transmission in North Central Nigeria. Pak J Biol Sci 14(4): 293-299.
- Cheesbrough M (2006) District laboratory practice in tropical countries, Cambridge University Press New York, USA.
- WHO (2010b) Basic malaria microscopy, part 1. Learners’ guide (2nd edn). WHO Press, Geneva, Switzerland.
- Agomo CO, Oyibo WA, Anorlu RI, Agomo PU (2009) Prevalence of malaria in pregnant women in Lagos, South-West, Nigeria. Korean J Parasitol 47(2): 179-183.
- Adesina KT, Balogun RO, Babatybde ASM, Sabbu MA, Fadeyi A, et al. (2009) Impact of malaria parasitaemia on haemaologic parameters in pregnant women at booking in Ilorin, Nigeria. Trends Med Res 4(4): 84-90.
- Nmadu PM, Peter E, Alexander P, Koggie AZ, Maikenti JI (2015) The prevalence of malaria in children between the ages of 2-15 visiting Gwarinpa general hospital Life-camp, Abuja, Nigeria. J of Health Sci 5(3): 47-51.
- Kunihya IZ, Samaila AB, Nassai I, Sarki A, Haruna MY (2016) Prevalence of malaria infection among children attending specialist hospital Yola, Adamawa State, Nigeria. J of Med and Biol Sci Res 2(8): 136-142.
- Nwaorgu OC, Orajaka, BN (2011) Prevalence of malaria among children 1-10 years old in communities in Awka North Local Government Area, Anambra State South East Nigeria. Afri Res Rev 5(5): 264-281.
- Okeke OP, Imakwu CA, Eyo JE, Okafor FC (2016) Prevalence of malaria infection in children in Anambra State, Nigeria after change of policy from presumptive/clinical to confirmed diagnosis. Ani Res Internat 13(1): 2385-2391.
- Nmorsi OPG, Isaac C, Ukwandu NCD, Ohaneme BA (2010) Pro-and anti-inflammatory cytokines Profiles among Nigerian children infected with Plasmodium falciparum Asian Pac J Trop Med 3(1): 41-44.
- Higgins SJ, Kain CK, Liles WC (2011) Immunopathogenesis of falciparum malaria: Implications for adjunctive therapy in the Management of severe and cerebral Malaria. Expert Rev Anti-Infect Ther 9 (9): 803-812.
- Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, (2005) Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: Cell signalling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem 280: 8606-8618.
- Akanmori BD, Kurtzhals JA, Goka BQ, Adabayeri V, Ofori MF, et al. (2000) Distinct patterns of cytokine regulation in discrete clinical forms of Plasmodium falciparum Eur Cytokine Netw 11(1):113-118.
- Hunt NH, Grau GE (2003) Cytokines: Accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol 24(9): 491-499.
- Langhorne J, Ndungu FM, Sponaas A, Marsh K (2008) Immunity to malaria: More questions than answers. Nat Immunol 9(7): 725-732.
- Jackobson PH, Mckay V, Morris-Jones SD, McGuire W, Van Hensbroek MB, et al. (1994) Increased concentrations of interleukin-6 and interleukin-1 receptor antagonist and decreased concentration of β-2 glycoprotein-1 in Gambian children with cerebral malaria. Infect Immun 62 (10): 4374-4379.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...