Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Case Report(ISSN: 2641-1725) 
Ciprofloxacin-Induced Cervical Spinal Stenosis and Upper Limb Paresis Post-Typhoid Fever: A Case Report Volume 5 - Issue 5
Yasser Mohammed Hassanain Elsayed*
- Critical Care Unit, Fraskour Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health (MOH), Damietta, Egypt
Received: November 23, 2020 Published: January 27, 2021
*Corresponding author: Yasser Mohammed Hassanain Elsayed, Critical Care Unit, Fraskour Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health (MOH), Damietta, Egypt
DOI: 10.32474/LOJMS.2021.05.000222
Abstract
Rationale: Typhoid (enteric) fever is one of the most serious infections worldwide. Drug-induced diseases is a vital issue in toxicology and clinical medicine. Ciprofloxacin is a fluoroquinolone antibiotic can cause a serious or irreversible disabling side including tendon, bone, muscles, joints, nerves, and central nervous system problems. Patient concerns: A middle-aged married male patient presented to the physician outpatient clinic with a typhoid fever progress to severe neck pain and weakness of both upper extremities.
Diagnosis: Ciprofloxacin-induced bilateral upper limb paresis and cervical spinal stenosis. Interventions: Magnetic resonance imaging Electrocardiography, Widal test, and decompressive surgical neck repair.
Outcomes: The deterioration after decompressive surgical neck repair had happened. Quadriplegia was a major complication.
Lessons: This is the first case that reports these adverse drug reactions with oral ciprofloxacin. Oral ciprofloxacin can induce bilateral upper limb paresis and cervical spinal stenosis. The identification of drug-induced disease is a pivotal step in the diagnosis decision making of any medical problems.
Keywords: Ciprofloxacin; drug-induced; typhoid fever; bilateral upper limb paresis; cervical spinal stenosis
Abbreviations: ECG: Electrocardiogram; MRI: Magnetic resonance imaging; VR: Ventricular rate
Introduction
Typhoid and paratyphoid (enteric) fever is a potentially serious
infective disease mostly, in developing countries1 Poor sanitation
and bad food hygiene are major risk factors [1,2]. It is caused by
Salmonella Typhi, Paratyphi A, Paratyphi B, and Paratyphi C2. The
usual incubation period is 7-14 days with a range of 3-60 days.
The infection is usually manifested with fever which increases
with disease progression, frontal headache, fatigue, muscular
pain, anorexia, and cough. Constipation, less frequent diarrhea,
abdominal pain, bradycardia, splenomegaly, and rose spots ‘rash
are other possible presentations1. The diagnosis of typhoid cannot
be confirmed based on symptoms and signs of the infection alone.
There is a wide variation in the symptoms of typhoid fever rather
than the broad differential diagnosis [3]. Serological markers and
bacterial culture with antigen discovery; and DNA intensification
are suggested tests [2]. Unfortunately, all of these are unacceptable
[2]. The Widal test measured the agglutinating antibodies against
LPS (O) and flagellar (H) antigens of Salmonella serovar Typhi in
the sera of in suspected cases of typhoid fever. It is an essential and
economic to perform is still widely used test [4,5]. Fluoroquinolones
(e.g., ciprofloxacin) and third generation cephalosporins (e.g.,
ceftriaxone) is used the initial antibiotics of choice1. Australian
guidelines recommend ciprofloxacin 500 mg orally, 12 hourly for
7-10 days [6]. Typhoid fever may be complicated with intestinal
bleeding, intestinal perforation, encephalopathy pancreatitis, heart failure endocarditis, myocarditis, liver failure, hepatitis or
pyelonephritis, glomerulonephritis, renal failure, pneumonia
from and respiratory failure, orchitis, arthritis and disseminated
intravascular coagulation [1,3]. The overall mortality rate is 10%
but it is less than 1% with adequate antibiotic therapy [1].
Ciprofloxacin is a fluoroquinolone broad-spectrum antibiotic
that is commonly used to treat different types of bacterial infections,
e.g., dermatitis, osteomyelitis and arthritis, sinusitis, pneumonia,
urinary tract infections, and infective diarrhea [7]. Ciprofloxacin
was patented in 1980 and introduced in 1987 [8]. It is on the World
Health Organization’s List of Essential Medicines [9]. It is active
against some Gram-positive and many Gram-negative bacteria [10].
It acts by inhibiting the type II topoisomerase (DNA gyrase) and
topoisomerase IV that are essential for bacterial DNA separation
and inhibiting the cell division [11]. Fluoroquinolone antibiotics
can cause serious or irreversible disabling side effects e.g., tendon
rupture and nerve problems7. So, ciprofloxacin adverse effects
are frequently including tendon, bone, muscles, joints, nerves, and
central nervous system problems [7,12]. Fluoroquinolone treatment
should be immediately ceased if a patient reports neuropsychiatric
side effects, tendons, muscles, joints adverse effects. The physician
should be switch to a non-fluoroquinolone antibiotic [7,9,13]. All
patients who receive a systemic fluoroquinolone should be made
aware of the potential for changes in memory, attention span, and
other psychiatric functions, and should report signs of alarming
CNS effects to a healthcare professional [13].
Aim of this study: In this manuscript, I reported the
development of cervical spinal stenosis and bilateral upper limb
paresis within 7 days after using ciprofloxacin in a middle-aged
male patient.
Case Presentation
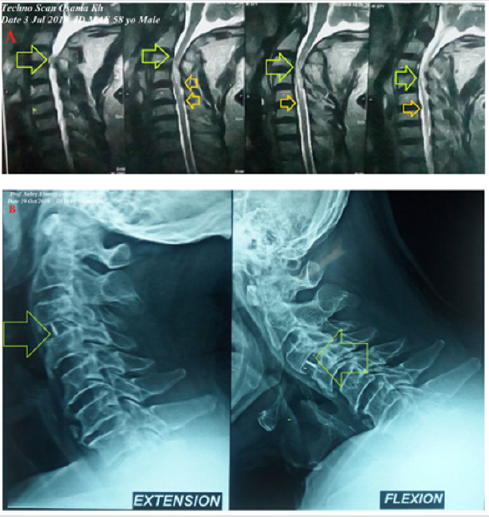
A 58-year-old married, farmer, Egyptian male patient presented to the physician outpatient clinic with palpitations, fever, and headache. The patient gave a history of constipation and abdominal pain. The patient denied a history of cardiac, thyroid, neurological, and musculoskeletal complain or other relevant diseases. Upon examination, the patient appeared sweaty, rigor, fatigued, and coated tongue. His vital signs were as follows: blood pressure of 100/70 mmHg, the pulse rate of 66/bpm; and regular, the respiratory rate of 32/min, the temperature of 39.8°C, and the pulse oximeter of oxygen (O2) saturation of 99%. No more relevant clinical data were noted during the clinical examination. The electrocardiogram (ECG) was done within 7 days of treatment which showed normal sinus rhythm at 76 beats/min (Figure 1). The direct agglutination test for Widal was positive for; Typhi (O); 1/160, Typhi (H); 1/640, Paratyphi (A); 1/160, Paratyphi (B); 1/320. Ciprofloxacin (oral tablet) 750 mg twice daily was prescribed. The patient started to complain of acute neck pain, shoulders pain, tingling, numbness, and weakness in both upper limbs. Symptoms was elicited after bending and twisting the patient neck (Spurling’s maneuver). Ciprofloxacin was immediately ceased. The patient referred to neurosurgeon for consultation. MRI film of the cervical spine was requested. It is showing marked cervical canal stenosis at C 3-4 level and mild cervical canal stenosis at C 4-5 level and at level C 5-6 level (Figure 2A). The neurosurgeon decided to make decompressive neck surgery. But, unfortunately, quadriplegia was the end result. The patient was managed conservatively. The investigations done were the troponin test, electrolyte level, thyroid studies, and random blood sugar with no detectable abnormal results. Complete blood count showed leucopenia. Within 15 days of decompressive neck surgery, Plain X-Ray film of the cervical spine on both extension and flexion view was done. It is showing evidence of cervical spine internal fixation at C 3-4 level (Figure 2B). Complete clinical characteristic of the patient on presentation and after treatment was summarized (Table 1).
Table 1: Summary of the clinical characteristic of the patient on presentation and after ciiprofloxacin.
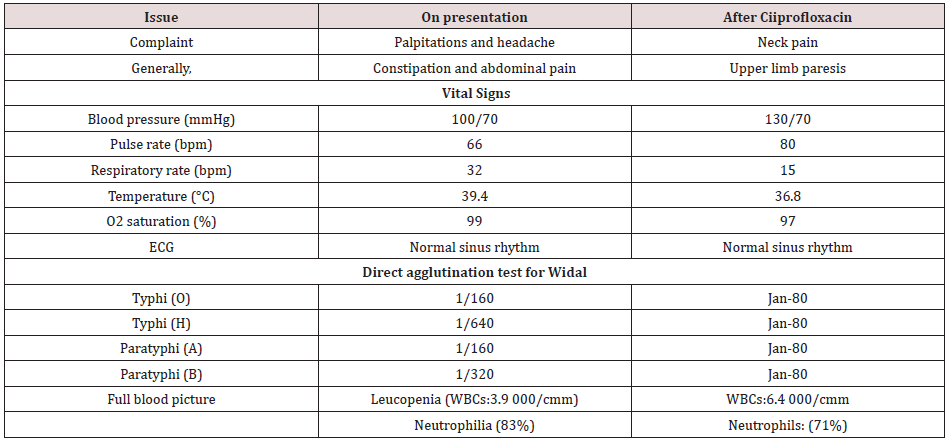
Figure 1: ECG tracing within 72 hours of treatment which showed normal sinus rhythm at 76 beats/min, Wander rhythm at V2 (green color) and left axis deviation.
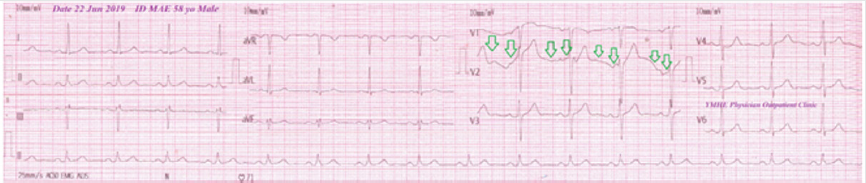
Figure 2: A. MRI film of the cervical spine showing marked cervical canal stenosis at C 3-4 level (lime arrows) and mild cervical canal stenosis at C 4-5 level and at level C 5-6 level (orange arrows). B. Plain X-Ray of the cervical spine on both extension and flexion view showing an evidence of cervical spine internal fixation at C 3-4 level (lime arrows).
Discussion
a) Overview: The current case is a middle-aged married
male patient presented to the physician outpatient clinic with
bilateral upper limb weakness within 7 days after using oral
ciprofloxacin in typhoid fever.
b) The primary objective for the current case study was the
presence of cervical spinal stenosis and bilateral upper limb
paresis within 7 days after using oral ciprofloxacin.
c) The secondary objective for the case study was How
would you manage cervical spinal stenosis and bilateral upper
limb paresis?
d) The main differential diagnosis for the study case is
cervical myelopathy.
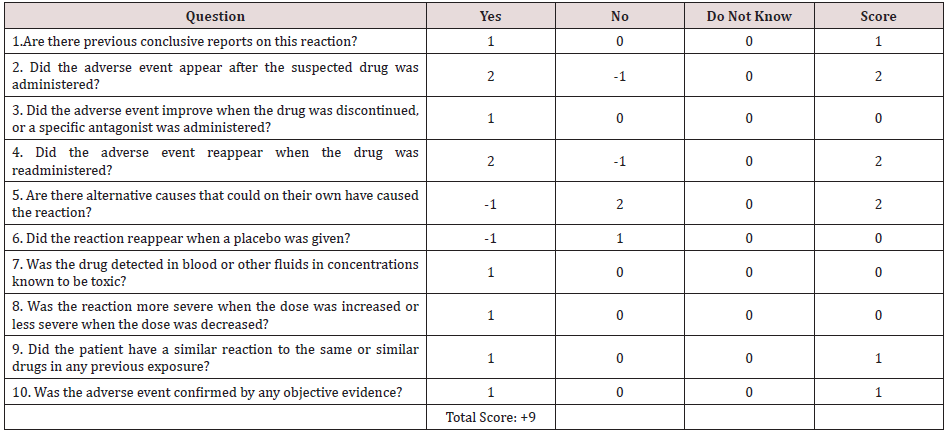
e) After the exclusion of other possible triggers in the current
case, the Naranjo probability scale was used to evaluates the
association between oral ciprofloxacin and development of
both cervical spinal stenosis and bilateral upper limb paresis.
Naranjo probability scale in the current case study was 9. It is
meaning that there was a definite relationship between these
adverse drug reactions and the causing drug; oral ciprofloxacin
(Table 2).
f) Finally, I reported the development of cervical spinal
stenosis and bilateral upper limb paresis within 7 days after
using oral ciprofloxacin in a 58-year-old male.
g) Indeed, the mechanism of oral ciprofloxacin inducing
cervical spinal stenosis and bilateral upper limb paresis is
unknown. The author thinks that the age may be a trigger factor.
The cartilaginous damage and spinal osteoarthritis may interpret
this complication.
h) This is the first case that reports these adverse drug
reactions with oral ciprofloxacin. So, I can’t compare this case with
another case because there was no similar publicized case report.
i) Despite the drug-drug interactions (DDIs) or even drugfood
interactions have a strong impact in inducing various serious
drug adverse effects, but it was unviable in my case report. Absent
of using drug combinations in the patient history may exclude the
theory of drug-drug interactions.
j) Drug-induced diseases is a pivotal step in the diagnosis
decision making of any medical problems.
k) Drug side effects are a sometimes-strong way for the
diagnostic challenge in clinical medicine.
Limitations of the study: There are no known limitations in the study.
Conclusions
a) Ciprofloxacin can induce bilateral upper limb paresis and
cervical spinal stenosis.
b) So, attention must be taken on using ciprofloxacin. to
reduce the risk of the development of these adverse drug
reactions.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
I wish to thank Dr. Yasser Rizk MD for his radiological consultation.
References
- Mayer CA, Neilson AA (2010) Typhoid and paratyphoid fever Prevention in travelers. Australian Family Physician 39(11): 847-851.
- Jabeen A, Yasin N, Khan H, Hussain M (2018) A review: Typhoid fever. J Bacteriol Infec Dis 2(2): 1-7.
- Mouton F, Ohuoba EI, Evans M, Desalu I (2017) Typhoid enteric fever–Part 1. Update in Anaesthesia 32: 13-16.
- Fadeel MA, House BL, Wasfy MM (2011) Evaluation of a newly developed ELISA against Widal, TUBEX-TF and Typhidot for typhoid fever surveillance. J Infect Dev Ctries 5: 169-175.
- Khan K, Khalid L, Wahid K (2017) Performance of TUBEX® TF in the diagnosis of enteric fever in private tertiary care Hospital Peshawar, Pakistan. J Pak Med Assoc 67: 661-664.
- (2006) Australian therapeutic Guidelines: Antibiotic. Version 13, Therapeutic Guidelines limited, Melbourne, USA.
- Durbin K (2020) Ciprofloxacin.
- Fischer Jnos, Ganellin C Robin (2006) Analogue-based Drug Discovery. John Wiley & Sons pp. 500.
- (2019) World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. Geneva.
- (2007) First aid for the USMLE step 2 CK, 6th McGraw-Hill Medical, USA.
- Drlica K, Zhao X (1997) DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiology and Molecular Biology Reviews 61(3): 377–392.
- Liu X, Ma J, Huang L, Zhu W, Yuan P, et al. (2017) Fluoroquinolones increase the risk of serious arrhythmias: A systematic review and meta-analysis. Medicine (Baltimore) 96(44): 8273.
- (2018) Food and Drug Administration. Safety Announcement: FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics; requires label changes.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...