Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6921
Opinion(ISSN: 2641-6921) 
Pyrazolo [1,5-A] Pyrimidines an Interesting Scaffold for Optical Applications Volume 4 - Issue 1
Alexis Tigreros1 and Jaime Portilla2*
- 1Post doctoral Researcher, Departamento de Química, Universidad de Los Andes, Bogotá DC, Colombia
- 2Associate Professor, Departamento de Química, Universidad de Los Andes, Bogotá DC, Colombia
Received: March 22, 2021; Published: April 01, 2021
*Corresponding author: Jaime Portilla, Associated Professor, Bioorganic Compounds Research Group, Department of Chemistry, Universidad de los Andes, Carrera 1 No. 18A-10, Bogotá, Colombia
DOI: 10.32474/MAMS.2021.04.000178
Opinion
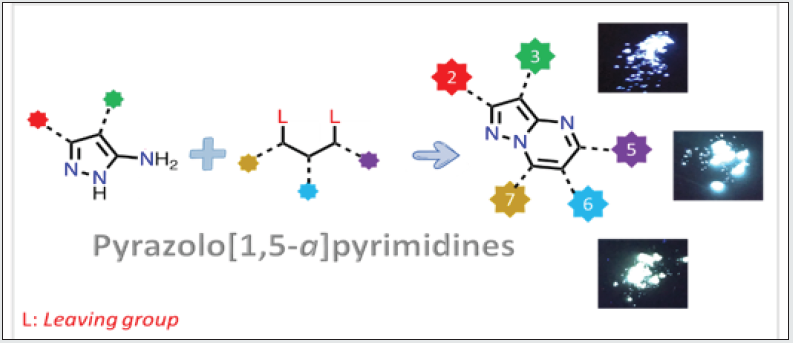
Organic fluorescent compounds are playing and will play an important role in scientific research in the next decades owing to their potential technological applications in the fields of chemo sensors, biological imaging, and optical devices [1]. With hundreds of metal-free dyes published every year an immediate question arises: is there any room for new organic fluorophores? To find an appropriate answer, we should analyze the present and past of organic fluorophores. On one hand, conventional organic fluorophores like pyrene, anthracene, or perylene are essentially non-fluorescent in the solid-state, which limits their usage in practical applications. On the other hand, more complex and modern organic dyes such as BODIPY, Rhodamine, or coumarin, to name a few, with exceptional optical properties both in solution and solid-state lack of good sustainable performance, with expensive manufacture processes and high amounts of wastes release during their production (Figure 1). Consequently, the answer to the above question is yes, as modern society we really need better fluorophores and thus, it is highly desirable to find modern alternatives that combine excellent photophysical performance with low-cost and efficient synthetic approaches. In this context, many reasons allow us to think that pyrazolo[1,5-a] pyrimidines, well-known for their wide applications in medicinal chemistry, emerge also as an important alternative for optical applications due to their proven synthetic versatility [1].
From theoretical and crystallographic studies, the structure of
pyrazolo[1,5-a] pyrimidines (PPs) have been found as a rigid and
highly planar nucleus, vital features for optoelectronic applications.
Moreover, the electronic communication with substituent on their
periphery depends on the position and the bulky and/or electronic
nature of the group attached. Thus, photophysical properties
can be tuned from highly efficient fluorescent compounds in
solution by using electron donating groups at position 7 to solidstate
high emitters with bulky substituents in the same position
[2]. Noticeable, most of the raw materials needed to produce
pyrazolo[1,5-a] pyrimidines are non-expensive and commercially
available compounds: amino pyrazoles, aldehydes, acetophenones,
dimethylformamide-dimethyl acetal (DMF-DMA), β-dicarbonyl
compounds or β-ketonitriles. Moreover, structural diversity in this
fused pyrazole can be accomplished during their synthesis or via
post-functionalization strategies. In general, the synthetic pathways
involve a cyclocondensation reaction between an aminopyrazole
with a 1,3-biselectrophilic system, in the absence of any metal
catalyst, with good overall yields. Interestingly, the π-excedent
pyrazolic ring in PPs is susceptible to electrophiles (position 3)
while the π-deficient pyrimidine moiety is attracted to nucleophiles
(positions 5 and 3). As a result, aromatic substitution reactions are
frequent that allows further functional group introduction through
well-known protocols like nitration (-NO2), formylation (-CHO),
halogenation (F, Cl, Br, or I), amination or coupling reactions.
Therefore, a huge number of functional groups combinations with
interesting biological and/or optical properties/applications can
be synthesized starting from relatively simple raw materials.
Attractive photophysical properties such as good absorption
coefficients (from 2001 to 39000 M-1 cm-1), quantum yields as high
as 98% in solution, and 63% at solid-state, excellent photostability,
and absorption and emission wavelength tunable with the
substituent nature in the periphery of the heterocyclic core,
indicate that interesting set of applications can be formulated for
this fluorophore. However, and for the best of our knowledge, just
a few examples have been reported for the optical application of
these compounds. For example, professor’s Jian Feng Ge group in
Suzhou (China) use a pyrazolo[1,5-a] pyrimidine-triphenylamine
hybrid system to study the lipid content in cancer cells, thanks to
their environment sensitive emission properties [3]. The same
principle was used in our research group to propose a preliminary
detection of water in organic solvents. Furthermore, we combined
pyrazolo[1,5-a] pyrimidine with two cyanide acceptor groups at
position 3, allowing the detection of this toxic anion (CN−) by using
UV-Vis and fluorescence techniques. In comparison with some other
organic compounds regularly used for optical applications, the
pyrazolo[1,5-a] pyrimidines have shown superior photostability,
lower raw-materials cost per gram, tunable emission intensities
in solution and/or solid-state, and higher reaction mass efficiency
(lower waste generation). Therefore, we hope these interesting
family of compounds will be useful alternatives for optical materials
in non-linear optics, organic light emitting devices (OLEDs), organic
solar cells, and chemo sensors field. By the time, we are working on
a variety of applications related to other interesting substrates and
are very excited about the results [3].
Acknowledgment
This research was supported by the Chemistry Department, Vicerrectoría de Investigaciones and Science Faculty (project INV- 2019-84-1800) at the Universidad de los Andes.
References
- Chan J, Dodani SC, Chang CJ (2012) Reaction-based small-molecule fluorescent probes for chemo selective bioimaging. Nat Chem 4(12): 973-984.
- Tigreros A, Aranzazu SL, Bravo NF, Zapata-Rivera J, Portilla J (2020) Pyrazolo [1,5-a] pyrimidines-based fluorophores: A comprehensive theoretical-experimental study. RSC Adv 10: 39542–39552.
- Yang XZ, Ru Sun, Xiao Guo, Xue-Rui Wei, Jing Gao, et al. (2020) The application of bioactive pyrazolopyrimidine unit for the construction of fluorescent biomarkers. Dye Pigment 173: 107878.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...