Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6921
Short Communication(ISSN: 2641-6921) 
In-Silico Evaluation Of Lamotrigine Schiff Base Of Cinnamonaldehyde And Its Metal Coordinates At Voltage Gated Sodium Channel Volume 4 - Issue 5
Saima Najm*
- Faculty of Pharmacy, Lahore College of Pharmaceutical Sciences, Lahore, Pakistan
Received: October 29, 2021; Published: November 12, 2021
*Corresponding author: Saima Najm, Faculty of Pharmacy, Lahore College of Pharmaceutical Sciences, Lahore, Pakistan
DOI: 10.32474/MAMS.2021.04.000199
Introduction
Lamotrigine belongs to a class of phenyltriazine compounds, chemically unrelated to other anticonvulsants [1]; used to control seizures and convulsions of various grades [2]. The antiepileptic effect of LTG entails from its binding with the voltage gated sodium channels (VNaC) and thus inhibiting the release of endogenous amino acids and acetylcholine [3,4]. Schiff bases of antiepileptic drugs protect against seizures through variety of cellular targets, like synaptic vesicle protein, neurotransmitter metabolic enzyme, neurotransmitter transporter and ion channels [5]. A homology model of VNaC was prepared for molecular docking studies of lamotrigine [6]. Docking of metal derived schiff base ligand is a new approach in computational chemistry; it predicts the binding affinity of small molecules with receptor that results in new complex with overall minimum energy [7,8].
Methodology /Molecular Modeling
Three dimensional (3D) crystal structure of the enzyme was retrieved from RCSB Protein Data Bank (PDB) with PDB-ID: 5kav (https://www.rcsb.org/structure/5KAV) for anticonvulsant activity. Two dimensional (2D) structures of lamotrigine schiff base metal complexes were drawn and optimized by ACD/Chem Sketch software and save as MDL file. The MDL files were 3D protonated and energy minimized to PDB by using Open Babel GUI [9]. Docking studies was carried out using Auto Dock 4.2 program [10]. The synthesis and structure of ligands were already reported by (Saima et al., 2021) in ACS Omega with 2KAV receptor. Now crystal structure of 5KAV was modelled using Auto Dock Tools 1.5.6; impurities were removed, whereas, partial charges and polar hydrogen were added. Macromolecule was saved in its respective PDBQT format for ligand interactions [11]. The best active region of enzyme was selected by targeting binding site with amino acid residues involved in binding to ligand [12]. The grid box was set at 100x100x100 Å along X, Y and Z axis with grid spacing of 0.375Å to recognize the binding site of ligand. The auto dock parameters used were: Genetic algorithm with population size = 150; Maximum number of energy evaluations = 250000; Genetic algorithm cross-over mode = 2 points. The rigidity parameters were set for receptor keeping ligand flexible. Ten docked orientations (poses) were obtained after protein-ligand docking at 5KAV receptor. The best conformation was screened in terms of lowest binding energy among several bioactive conformations generated by various interactions. Cluster analysis of protein binding sites with lowest binding energy was further explored using Pymol Molecular Graphics System [2,8]. The reliability of docking program was validated by using re-docking method; root mean square deviation (RMSD) was then calculated and in all cases RMSD value of <2.0 Å was considered accurate in predicting binding orientation of ligand [13].
Results and Discussion
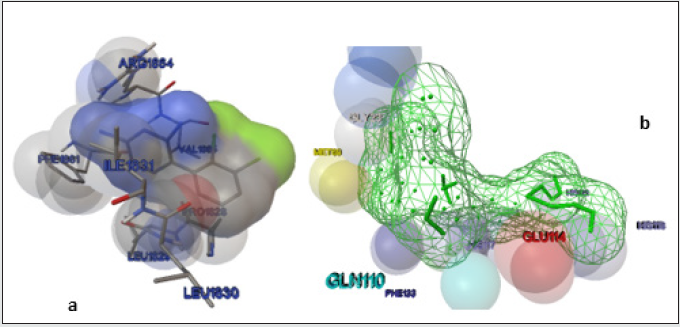
Previous mechanistic investigations have provided evidence that the anti-epileptic effects of LTG originates from its binding at the voltage gated sodium channel [6]. LTG specifically block the sodium channel by binding to the pore in the inactivated open state. Therefore, we intended that the newly synthesized LTGSB- M complexes must bind to the VNaC to elicit the anti-epileptic effect. To test this hypothesis, we docked the LTG-SB-M complex using co-crystal structure of human VNaC (PDB ID: 5KAV) for calculating binding interactions. The detailed results of LTG-SB-M complex docked with 5KAV were tabulated in Table 1; it is cleared from table 1 that compound 7c shows good interaction at VNaC. The interacting residues of LTG as standard and 7c were also demonstrated in (Figure 1) (a) and (b) respectively.
Figure 1: (a) Docked structure of LTG with modelled 5KAV receptor by Auto Dock; together with amino acid residues at the binding site of 5KAV. (b) Wire frame view of 7c on 5KAV receptor, generated by Auto Dock, along with interacting residues in the binding pocket of 5KAV.
Conclusion
In this study it is confirmed that LTG-SB-M complexes synthesized from cinnamon aldehyde shows good binding energies at VNaC and hence can be used as antiepileptic drugs in future.
References
- Najib FM, Mustafa MS (2014) Spectrophotometric methods for simultaneous determination of carbamazepine and lamotrigine in binary mixtures and urine samples. Mal J Anal Sci 18: 491-506.
- Poureshghi F, Ghandforoushan P, Safarnejad A, Soltani S (2017) Interaction of an antiepileptic drug, lamotrigine with human serum albumin (HSA): application of spectroscopic techniques and molecular modeling methods. J Photochem Photobio 166: 187-192.
- Vallés AS, Garbus I, Barrantes FJ (2007) Lamotrigine is an open-channel blocker of the nicotinic acetylcholine receptor. Neuroreport. 18: 45-50.
- Kim KJ, Jeun SH, Sung KW (2017) Lamotrigine, an antiepileptic drug, inhibits 5-HT3 receptor currents in NCB-20 neuroblastoma cells. Kor J Physio Pharmcol 21: 169-177.
- Robert MAR (2010) Cellular effect of antiepileptic drugs. Bepress 1(1): 1-40.
- Cronin RNB, Duclohier A, Wallace H (2003) Binding of the anticonvulsant drug lamotrigine and the neurotoxin batrachotoxin to voltage-gated sodium channels induces conformational changes associated with block and steady-state activation. J Bio Chem 278(1): 10675-10682.
- Sankar JN, Arun VK (2012) Synthesis and docking studies of Schiff bases derived from 4-Aminopyridine. J Sci Inno 1(1): 9-11.
- Mahmoud WH, Deghadi RG, Mohamed GG (2020) Metal complexes of ferrocenyl-substituted Schiff base: Preparation, characterization, molecular structure, molecular docking studies and biological investigation. J Organometal Chem 2: 1-42.
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, et al. (2009) AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comp Chem 30: 2785-2791.
- Garrett MM, Goodsell DS, Robert SH, Ruth H, William EH, et al. (1998) Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J Comp Chem 19: 1639-1662.
- Yang J, Roy A, Zhang Y (2013) Protein-ligand binding site recognition using complementary binding specific substructure comparison and sequence profile alignment. J Bioinfo 29: 2588-2595.
- Ebrahimipoura SY, Sheikhshoaiea I, Castrob J, Dusekc M, Tohidiana Z, et al. (2015) Synthesis, spectral characterization, structure studies, molecular docking and antimicrobial evaluation of new dioxidouranium(VI) complexes incorporating tetradentate N2O2 Schiff base ligands. RSC Adv 9: 13-57.
- Jabeen M, Ahmad S, Shahid K, Sadiq A, Rashid U (2018) Ursolic Acid Hydrazide Based Organometallic Complexes: Synthesis, Characterization, Antibacterial, Antioxidant, and Docking Studies. J Fron Chem 6: 55-65.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...