Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6921
Research Article(ISSN: 2641-6921) 
Evaluation of Physicochemical Parameters and Heavy Metals of Wastewater Used for Irrigation at Tukuntawa Radio Station, Kano Volume 3 - Issue 3
Muhammad M*1, Gwarzo FS2, Baso SA3 and Garba S1
- 1Department of Integrated Science, School of Science Education, Saadatu Rimi College of Education, Nigeria
- 2Department of Biology, College of Arts, Science and Remedial Studies, Nigeria
- 3Department of Chemistry, School of Science Education, Saadatu Rimi College of Education, Nigeria
Received: October 22, 2020; Published: November 05, 2020
*Corresponding author: Muhammad M, Department of Integrated Science, School of Science Education, Saadatu Rimi College of Education, Kumbotso, Nigeria
DOI: 10.32474/MAMS.2020.03.000164
Abstract
Evaluation of physicochemical parameters and heavy metals concentration of wastewater used for irrigation at Tukuntawa Radio Station was carried out. Six samples were collected from different sampling points. Levels of various physicochemical parameters such as Temperature, pH, Electrical conductivity (EC), Total Dissolved Solids (TDS), turbidity, F-, and PO3- were determined using standard analytical methods. The concentration of, Fe, Cu, Cr and Mn were determined using standard methods. The parameters analyzed are within the maximum permissible limits (MPL) recommended by Nigerian Standard for Drinking Water Quality (NSDWQ) and world Health Organization (WHO) with exception of turbidity level at all the sampling station, EC concentration at A, D, and F sampling point as well as TDS concentrations at D sampling point. The results also showed that, the concentration of Cr, Cu, Fe, and Mn in some sampling sites were slightly above the maximum permissible limit (MPL) recommended by NSDWQ and WHO.
Keywords: Heavy metals; irrigation; physicochemical parameters; wastewater; world health organization
Introduction
Water is an essential component of the environment that sustains life on the earth, and this makes all organisms depend on water for their survival [1,2]. Despite the importance of water, there are many factors that changes the quality of water because of urbanization, industrialization, population growth, geological factors among others. According to [3], rapid progress made in industrialization without adequate environmental safety measures lead to pollution of water, which, in turn, results in lack of good quality water both for irrigation and drinking purpose. Akpan-Idiok et al. [4] reported that, the assessment and continuous monitoring of water quality sourced from rivers and other bodies can be used to define existing conditions, detect, trend and/or establish sources of water pollution. Concentration and composition of dissolved constituents in water determine its suitability for irrigation use. Suitability of water for irrigation purposes depended on the effect of some mineral constituents in the water on both the soil and the plant [5]. Reference [6-8] observed that important factors that influence the irrigation water quality are salt and sodium concentrations as represented by EC, Sodium Adsorption Ratio and Residual Sodium Carbonate. Abdullahi et al. [9] showed that, the primary source of heavy metals in irrigation and drainage canals is because of discharge of domestic waste waters which contain high concentrations of metals such as copper, iron, lead, and zinc. These heavy metals are derived from household products such as cleaning materials, toothpaste, cosmetics and human faeces. Uzairu et al. [10] and Asonye et at. [11] reported that, pollution of water with heavy metals is of grave consequence as it affects both terrestrial and aquatic lives. It may cause disease due to the presence of some hazardous substance which may distort the water quality, add odours, and significantly hinder economic activities. Therefore, there is needed to analyses the status of the water source use for irrigation within our localities. The objective of this research is to evaluate the physicochemical parameters and heavy metals of wastewater used for irrigation from Tukuntawa radio station in Kano metropolitan area.
Materials and Methods
In the preparation of reagents chemicals of analytical Analar grade were used with deionized water. All glass wares were cleaned and rinsed with detergents and immersed in 25% nitric acid and finally rinsed with de ionized water [12].
Sample collection
Samples of water were collected from six different locations at Tukuntawa Radio station in a clean container. The water samples were transported to Tamburawa water quality control laboratory less than 2 hours of collection and analysed within 24 hours. The sampling was carried out between the hour 9:30 am and 12:00am.
Determination of physicochemical parameters
The parameters analyzed were pH, temperature, EC, TDS, Turbidity, Fluoride, phosphate and heavy metals concentration. Standard analytical methods were adopted for determining the above-mentioned parameters [13,14].
Results
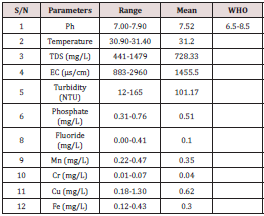
The result of various parameters analysed at all the six sampling sites were presented in Table 1 below. The results obtained were compared with the WHO and NSDWQ.
Discussion
The results obtained revealed that about 50% of the samples had conductivity level above maximum contaminant level of 1200 μscm-1 [15-17] as shown in Table above. However, the high conductivity level at the sites mentioned could be linked to sewage materials, leaching of inorganic contaminants as observed by [12]. The total concentration of soluble salts in irrigation water can be expressed in terms of electrical conductivity for purposes of diagnosis and classification. In general, water having conductivity below 750 μmhos/cm is satisfactory for irrigation. Water having a range of 750 to 2250 μmhos/cm is widely used, and satisfactory crop growth is obtained under good management and favorable drainage system [5]. TDS denotes various types of minerals present in the water in dissolved forms [18]. The total dissolved solid was analyzed and it can be observed that water from all the sampling sites had total dissolved solid levels below WHO maximum contaminant levels of 1000 mg/l [19] with exception of sampling site D which was found to be 1467 mg/l. The higher total dissolved solid reduce water clarity, which could contribute to the decrease in photosynthetic activities and might lead to an increase in water temperature. pH has direct and indirect effects on photosynthetic events and growth of water plant [20,21]. pH levels in the water samples analysed are presented in table above. The results obtained from all the sampling sites had pH level within the recommended value of 6.5-8.5 [12,15,17,19]. Madhuri [22] reported pH value slightly above current value.
According to [23], Turbidity is the suspension of particles in water interfering with the passage of light and is caused by a wide variety of suspended matter that range in size from colloidal to coarse dispersion depending on the degree of turbulence and may include inorganic and organic substances. The turbidity level in all the sample sites determined, showed that they are above the recommended levels of 5 NTU [15,19] as presented in Table 1 above. Higher turbidity in water could be due to Suspended materials, bacteria, plankton and dissolved organic and inorganic substance and higher turbidity is associated with Surface water sources as opined by [12]. The occurrence of Phosphate in water bodies is as result of domestic waste, detergent and agricultural runoff containing fertilizer [24]. The significance of phosphorus is principally regarding the phenomenon of over-enrichment of lakes and, to a lesser extent, rivers. Phosphorus gaining access to underground water bodies is perhaps unlikely [24]. The phosphate levels range between 0.31-0.76 mg/l with sample from site B being the least and site D the highest. Fluoride occurs naturally in quite rare instances; it arises almost exclusively from fluoridation of public water supplies and from industrial discharges [25]. Health studies have shown that the addition of fluoride to water supplies in levels above 0.6 mg/dm3. The fluoride levels for the water samples under investigation are within safe limits. The heavy metals analysed were Cr, Cu, Fe and Mn. From the results all the samples were within the safe limit recommended by [26] with exception of Cu, Fe at sampling site D. The concentration of Mn at all the sampling sites were found to be higher than the MPL of 0.05 mg/l as recommended by [15]. This may be because of human activities as well as industrial activities within the study area.
Conclusion
Physicochemical and heavy metals assessment of different samples from domestic and industrial effluents used for irrigation around Tukuntawa Radio station in Kano municipal local government area were carried out. Most of the physical parameters are within the NSDWQ and WHO safe limit. The levels of turbidity and TDS at all the sampling sites were higher than the recommended limits. Also, the level of EC at sampling sites A, D, and F were found to be higher. The concentration of heavy metals was also found to be within the recommended levels set by NSDWQ with exception of Cu and Fe at sampling point D, and Mn at all the sampling stations. The results of the analysis indicated that EC and TDS are related to one another.
Recommendations
The results of this analysis indicated that, parameters like TDS, turbidity, EC, Fe, Mn, and Cu in some sample analyzed showed higher concentration above the WHO and NSDWQ contaminant level especially the samples from tannery industry which is believed to be contaminated, the following steps may lower the concentration: Proper disposal of waste should be practice within the study area and beyond. Agencies own by Government and non-governmental should provide a means of educating the communities and industries on proper disposal waste. Further, analysis should be carried out to assess microbial, radionuclide, other organic and inorganic contaminants. Important Techniques like phytoremediation and bioremediation can be used to regulate the levels of the heavy metals in the wastewater from the study area. Regular monitoring of the water from the study area should be perform time-time.
References
- Qureshimatva UM, Maurya RR, Gamit SB, Patel RD, Solanki HA (2015) Determination of Physico-Chemical Parameters and Water Quality Index (WQI) of Chandlodia Lake, Ahmedabad, Gujarat, India. Journal of Environmental and Analytical Toxicology 5(4): 1-6.
- Smitha PG, Byrappa K, Ramaswamy SN (2007) Physicochemical characteristics of water samples of Bantwal Taluk, South-Western Karnataka, India. Journal of Environmental Biology 28(3): 591-595.
- Periyasamy M, Rajan MR (2009) Physicochemical Characteristics and Water Quality Index of Electroplating Industry Effluent. Journal of Industrial Pollution Control 25(1): 1-8.
- Akpan-Idiok UA, Ibrahim A, Udo IA (2012) Water quality assessment of Okpauku River for drinking and irrigation uses in Yala, Cross river state, Nigeria. Research Journal of Environmental Sciences 6(6): 210-221.
- Kouhsari N, Nagarajub D, Balasubramaniana A (2018) Groundwater quality assessment for irrigation purpose in Periyapatna Taluk, Mysuru district, Karnataka, India. International Journal of Research Science and Management 5(2): 67-89.
- Kundu S (2012) Assessment of Surface Water Quality for Drinking and Irrigation Purposes: A Case Study of Ghaggar River System Surface Waters. Bulletin of Environment, Pharmacology and Life Sciences 1(2): 01-06.
- Prasad MSVKV, Siva PG, Prasada PVVR (2015) Assessment of groundwater suitability for irrigation purpose: a case study of Narsapur-Mogalthur mandals, West Godavari district, Andhra Pradesh, India. IOSR Journal of Environmental Science, Toxicology and Food Technology 9(3): 07-11.
- Singh AK (2002) Quality Assessment of surface and subsurface water of Damodar river basin. Indian Journal of Environmental Health 44 (1): 41-49.
- Abdullahi MS, Uzairu A, Okunola OJ, Balarabe ML (2015) Risk Assessment of Metals in Irrigated Food Crops Grown along the Bank of Tungan Kawo Dam, Kontagora, Nigeria. IOSR Journal of Applied Chemistry (IOSR-JAC) 8(5): 53-59.
- Uzairu A, Okunola OJ, Wakawa RJ, Adewusi SG (2014) Bioavailability Studies of Metals in Surface Water of River Challawa, Nigeria. Journal of Applied Chemistry.
- Asonye CC, Okolie NP, Okenwa EE, Iwuanyanwu UG (2007) Some physico-chemical characteristics and heavymetal profiles of Nigerian rivers, streams and waterways. African Journal of Biotechnology 6(5): 617-624.
- Saeed MD, Mahmoud AM (2014) Determination of Some Physicochemical Parameters and Some Heavy Metals in Boreholes from Fagge G.A of Kano Metropolis Kano State Nigeria. World Journal of Analytical Chemistry 2(2): 42-46.
- Singh SN, Srivastav G, Bhatt A (2012) Physicochemical Determination of Pollutants in Wastewater in Dheradun. Current World Environment 7(1): 133-138.
- APHA (1998) Standard methods for the examination of water and wastewater. American Public health Association, Washington, DC, USA 18th Edition 45-60.
- NSDWQ (2007) Nigerian Standard for Drinking Water Quality. NIS 554, Son, Lagos, USA p. 30.
- WHO (2004) Guidelines for Drinking Water Quality. WHO, Geneva, Switzerland, USA 3rd
- USEPA (2003) US Environmental Protection Agency safe drinking Water act. EPA 816-F-03-016.
- Tiwari P (2017) Water Quality Assessment for Drinking and Irrigation Purpose. Indian Journal of Scientific Research 13(2): 140-142.
- WHO (2006) Guidelines for Drinking Water quality. 3rd Edition, WHO Press, Geneva, USA p. 398.
- Lena M, Gunasekaran C, Shobana G, Agnes AD, Mohana P, et al. (2012) Assessment of Physicochemical Parameters of Water in Different Ecosystems of Yercaud Hills, Eastern Ghats, South India. World Journal of Fish and Marine Sciences 4(3): 308-312.
- Kara Y, Kara I, Basaran V (2004) Investigation of some physical and chemical parameters of water in the Lake Isykli in Denizli, Turkey. International Journal of Agriculture and Biology p. 275-277.
- Madhuri SP (2017) Analysis of Soil and Wastewater Quality Used for Irrigation of Hated Mali Waster Watershed Area Near Kuwarkheda Village Near Jalgaon District Maharashtra, India. International Journal of Current Advanced Research 6(9): 6040-6041.
- Suleiman FB, Audu AA (2014) Analysis of Water from some Dams in Katsina State, Nigeria. IOSR Journal of Applied Chemistry 7(1): 01-09.
- Lodh R, Paul R, Karmakar B, Das MK (2014) Physicochemical studies of water quality with special reference to ancient lakes of Udaipur City, Tripura, India. International Journal of Scientific and Research Publications 4(6): 1-9.
- Chindo IY, Karu E, Ziyok I, Amanki ED (2013) Physicochemical Analysis of Groundwater of Selected Areas of Dass and Ganjuwa local Government Areas, Bauchi State Nigeria. World Journal of Analytical Chemistry 1(4): 73-79.
- WHO (1993) Guidelines for Drinking Water Quality. WHO, Geneva, Switzerland, 2nd

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...