Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4544
Short Communication(ISSN: 2637-4544) 
The Vitiated Ossein-Bacterial Osteomyelitis Volume 4 - Issue 5
Anubha Bajaj*
- Consultant Histopathologist, AB Diagnostics, India
Received:October 8, 2021Published: October 19, 2021
Corresponding author: Anubha Bajaj, Consultant Histopathologist, AB Diagnostics, India
DOI: 10.32474/IGWHC.2021.04.000199
Abstract
Infection and inflammation of the bone, initially designated as osteomyelitis by Auguste Nelaton in 1844, occurs due to diverse
pyogenic organisms, bacteria, fungi, and mycobacteria. The infection may extend to encompassing tissue and occurs as an acute or
chronic inflammation.
Osteomyelitis is categorized into acute osteomyelitis, subacute osteomyelitis, or chronic osteomyelitis contingent to clinical
duration of disease. Bacterial infection induces pyogenic osteomyelitis, a variant which is exceptionally delineated with the advent
of contemporary antibiotics. Following the articulation of a sinus tract, an enlarged epidermal inclusion cyst layered with stratified
squamous epithelium may be configured within the incriminated bone. Infrequently, the lining epithelium metamorphoses into
well differentiated squamous cell carcinoma, a malignancy which is accompanied by a superior prognosis.
Disease Characteristics
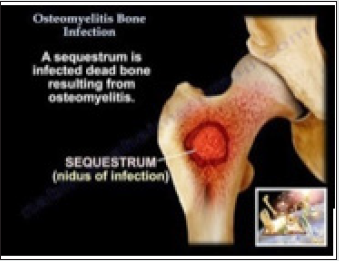
Significant bacterial ingress due to trauma, ischemia or foreign bodies with adherent microorganisms may infiltrate sites of exposed bone and engender osteomyelitis. An estimated 50% instances may occur due to unknown bacteria [1,2]. Osteomyelitis is generally detected between 2 years to 12 years although no age of disease emergence is exempt. A male predominance is exhibited with a male to female proportion of 3:1 [1,2]. Frequently the lower extremities, vertebral column, radial styloid and sacroiliac joints are incriminated. Location of infection is contingent to age of incriminated individual as altered vascularization is enunciated in diverse bone segments. Commonly, metaphysis or epiphysis in neonates, metaphyseal region in children and epiphyses or subchondral region of adult subjects are implicated in Figure 1. Distal and proximal femur, proximal tibia, humerus and distal radius are commonly implicated in children [1,2] Of undefined incidence, osteomyelitis displays an enhanced prevalence in male subjects of increasing age associated with comorbid conditions such as diabetes mellitus and peripheral vascular disease. Individuals with traumatic aetiology, bacterial dissemination, endocarditis, intravenous drug abusers and open fractures exhibit an enhanced possible occurrence of osteomyelitis [1,2]. Also, peripheral vascular disease, peripheral neuropathy, orthopedic implants, chronic or inadequately healed wounds occurring in diabetes mellitus enunciate an enhanced possibility of osteomyelitis [1,2].
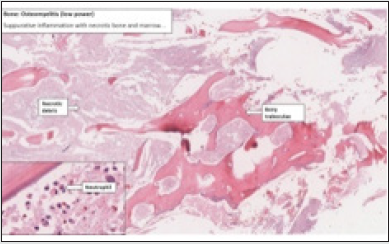
Figure 1: Osteomyelitis with the frequently discerned hematogenous bacterial dissemination encountered in children [10].
Disease Pathogenesis
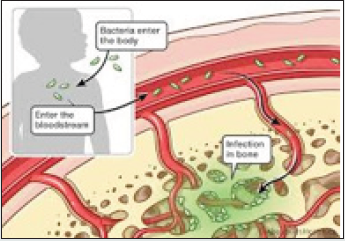
Bacterial proliferation occurring within bone may engender inflammation and necrosis which disseminates through the Haversian system or medullary canal within the bone shaft and involves the periosteum in Figure 2. Sub-periosteal abscess may impair vascular perfusion, thereby inducing additional necrosis and draining sinuses [3,4]. Acute and chronic osteomyelitis can be classified contingent to duration of illness or mechanism of infection as haematogenous dissemination or contiguous infection [3,4]. Bone infection may ensue through the haematogenous route with distant bacteria infiltrating the bone wherein osteomyelitis is essentially engendered from a singular microorganism. Haematogenous osteomyelitis is frequently discerned within metaphyseal region of long bones in children or flat bones or vertebral column in adults [3,4]. Direct inoculation of bone may be traumatic or iatrogenic and appears with ulceration or surgical inoculation. Osteomyelitis engendered with direct bacterial inoculation is delineated with open fractures, bone reconstructive surgery or with insertion of orthopedic implants [3,4].
Figure 2: Osteomyelitis depicting dissemination of neutrophils and lymphocytes commingled with foci of necrotic bone [11].
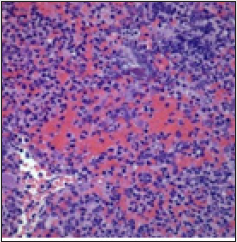
Bacterial dissemination in contiguous osteomyelitis ensues from joints or circumscribing soft tissue and may incriminate the vertebral column in adults. Contiguous osteomyelitis is infrequent and may be induced by singular or multiple micro-organisms. Contiguous infection is subdivided according to presence or absence of associated vascular insufficiency [3,4]. Contiguous osteomyelitis arising due to trauma, surgical intervention, immune deficiency or intravenous drug abuse occurs in young adults in Figure 3. Contiguous osteomyelitis occurring due to trauma is associated with infected, exposed soft tissue and cutaneous surfaces [3,4]. Decubitus ulcer, urinary tract infection, diabetes and infected joint arthroplasty may engender contiguous osteomyelitis in older individuals. Contiguous osteomyelitis frequently appears in debilitated, wheelchair or bedbound subjects prone to pressuresores or cutaneous ulceration [3,4].
Figure 3: Osteomyelitis delineating scattered neutrophils and marrow spaces intermingled with foci of osteonecrosis [12].
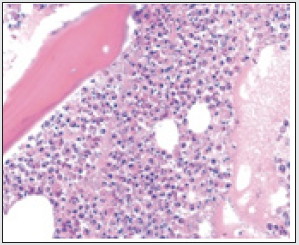
Lesions are commonly discerned in the sacrum, gluteal region and heel. Frequently, osteomyelitis occurs due to vascular insufficiency in concurrence with diabetes mellitus. Diabetic subjects depict compromised vascular perfusion within the lower extremities which impairs local immunity and healing of cutaneous wounds and stimulates dissemination of infection. Sensory neuropathy engenders cutaneous ulceration in diabetes mellitus, thus aggravating bacterial influx in Figure 4. Typically, polymicrobial flora from cutaneous surfaces or gastrointestinal tract accumulate within the ulcers wherein soft tissue infection may rapidly disseminate to underlying bone [3,4]. Staphylococcus aureus can thrive within intracellular environment, manifests receptors such as adhesins and may adhere to bone matrix constituents as laminin, collagen, fibronectin, or bone sialo glycoprotein [3,4].
Figure 4: Osteomyelitis enunciating sequestrum, inflammatory cells and exudate admixed with necrotic debris [13].
Staphylococcus aureus formulates collagen-binding adhesin
which initiates adherence to bone cartilage. Also, fibronectinbinding
adhesion aids bacterial attachment to surgical implants.
Bacteria may be layered within a protective biofilm coating the
underlying surface. Aforesaid features of bacterial adherence
contribute to phenotypic resistance towards antibiotic therapy
and aid intracellular survival with persistence of bone infections
and consequent failure of short-term antibiotic therapy [5,6].
Osteomyelitis engendering pathogens are contingent to age
of incriminated individual. Staphylococcus aureus, Brucella
or Salmonella spp may contribute to acute haematogenous
osteomyelitis arising in adults and children [5,6].
Also, methicillin-resistant Staphylococcus aureus (MRSA),
coagulase-negative Staphylococci, beta-haemolytic Streptococci,
Enterococci, aerobic gram-negative bacilli such as Enterobacter spp,
Pseudomonas spp, Escherichia coli, Klebsiella spp, Haemophilus
influenza, Treponema, Listeria spp and anaerobic gram-negative
bacilli such as Peptostreptococcus, Clostridium spp, Bacteroides
may engender from osteomyelitis [5,6]. Infrequently, pathogens
such as Bartonella henselae, Mycobacterium tuberculosis,
nontuberculous mycobacteria as Mycobacterium avium
intracellulare, fungi such as Candida spp, Blastomyces, Coccidiodes,
Cryptococcus and Aspergillus may induce osteomyelitis, especially
in immunocompromised individuals [5,6]. Inoculation with
Actinomyces or Sporothrix occurs with trauma. Pasteurella
multocida or Eikenella corrodens are isolated from human or
animal bites. Occasionally, osteomyelitis may be associated with
malakoplakia [5,6].
Clinical Elucidation
Anatomic subcategories of osteomyelitis are
a) Stage I where osteomyelitis is confined to bone medulla
b) Stage II with superficial disease
c) Stage III with localized disease
d) Stage IV with diffuse osteomyelitis [5,6]
Associated comorbidities exemplifying compromised host
immunity are malnutrition, renal or hepatic failure, diabetes
mellitus, chronic hypoxia, neoplasia and immunodeficiency
disorders [5,6]. In Figure 5 Localized factors contributing to
decimated immunity are chronic lymphedema, venous stasis,
vascular disease implicating major or miniature blood vessels,
arteritis, peripheral neuropathy and tobacco consumption [5,6].
Clinical representation of osteomyelitis is contingent to disease
a etiology. Acute osteomyelitis manifests gradually within few days
to two weeks. Localized symptoms such as erythema, swelling,
warmth upon incriminated site, dull pain at rest or upon mobility
and constitutional symptoms as fever or chills may appear. Acute
osteomyelitis may enunciate septic arthritis, especially of bone
metaphysis impacted within infected joint capsule. Septic arthritis
of elbow, shoulder and hip joint may complicate osteomyelitis
arising within proximal radius, humerus or femur [5,6]. Deepseated,
extensive, non -healing ulcers may be discerned despite
appropriate therapy, especially in diabetics or debilitated subjects
[6].
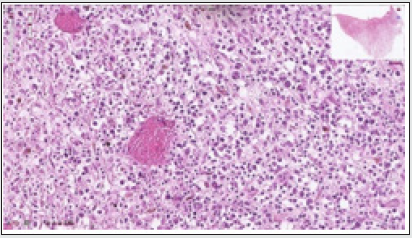
Figure 5: Osteomyelitis exemplifying dissemination of neutrophils and lymphocytes with fragments of necrotic bone [14].
Histological Elucidation
Upon gross examination, infants depict permanent joint and
epiphyseal deterioration with exclusion of metaphysis and diaphysis
whereas young children display metaphyseal damage with joint
exclusion. Adults demonstrate joint infection with extensive
incrimination of bone [7,8]. Acute osteomyelitis exemplifies
dissemination of inflammatory exudate through the bone with
periosteal elevation. An attenuated rim of reactive, periosteal bone
encompasses the centric, necrotic bone trabeculae in Figure 6.
Neonates demonstrate significant sub-periosteal accumulation of
inflammatory exudate [7,8]. Initially, bacterial propagation triggers
a localized inflammatory reaction engendering limited cellular
demise. Gradually, infection is segregated by peripheral granulation
tissue and deposition of new bone [7,8]. As bone metaphysis is well
perfused and depicts minimal proportion of functioning phagocytes,
metaphysis remains a common site of infection in hematogenous
osteomyelitis of long bones [7,8].
Sluggish vascular perfusion of metaphysis contributes to
microbial accumulation and infection. Enzymes from incipient
phagocytes lyse bone and circumvent microbial dissemination,
thereby initiating an inflammatory response which configures
pus, or a protein-rich exudate constituted of necrotic phagocytes,
tissue debris and microorganisms [7,8]. The inflammatory exudate
elevates intramedullary pressure and may rupture through bone
cortex or periosteum [8]. Upon microscopy, a significant neutrophilic
exudate is observed along with lymphocytes and plasma cells. Foci
of bone necrosis are delineated along with configuration of reactive,
new bone. Capillary proliferation is accompanied by fibrosis. Bone
marrow space may be replaced by inflammatory cells and exudate
[7,8]. Infection with Salmonella may demonstrate tuberculoid,
epithelioid cell granulomas with variable, centric necrosis [8].
Discontinuity of periosteum impairs periosteal vascular
perfusion with consequent bone ischemia and necrosis. Necrotic
bone is designated as “sequestrum”, is segregated from viable bone
by granulation tissue and may infrequently depict accumulation
of inflammatory exudate [8]. New bone deposited upon injured
periosteum is denominated as “involucrum” and it may partially
circumscribe the sequestrum in Figure 7. Exudate discharging from
sequestrum may configure a sinus tract, layered with stratified
squamous epithelium [7,8]. On microscopy, acute bacterial
osteomyelitis exhibits accumulated microorganisms, congested
or thrombosed vascular articulations and neutrophilic infiltration
[7,8].
Figure 7: Osteomyelitis exhibiting aggregates of neutrophils and lymphocytes intermixed with foci of dead bone [15].
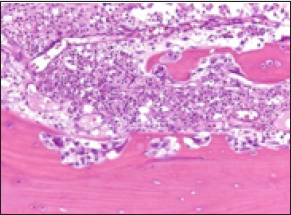
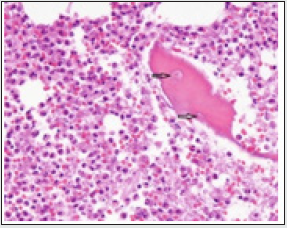
Figure 8: Osteomyelitis demonstrating aggregates of acute inflammatory cells as neutrophils and lymphocytes admixed with necrotic bone and red cell extravasation [16].
Contingent to histological manifestations, osteomyelitis is categorized as
• acute osteomyelitis which indicates bone infection preceding
genesis of sequestrum and usually appears within two weeks
of disease onset.
• chronic osteomyelitis denominates the development of
necrotic bone and sequestrum [7,8].
Differential Diagnosis
Osteomyelitis requires a segregation from conditions such as Charcot’s arthropathy, SAPHO syndrome constituted of synovitis, acne, pustulosis, hyperostosis and osteitis, arthritis or rheumatoid arthritis, metastatic bone disease with primary malignancy arising from Ewing’s sarcoma, osteosarcoma, lymphoma, multiple myeloma, pathological fracture or stress fracture, gout, avascular necrosis of bone, Langerhans cell histiocytosis (LCH), bursitis or sickle cell vaso-occlusive pain crises [1,2].
Investigative Assay
Competent clinical history and physical examination is essential and beneficial. Physical examination necessitates detection of an infective nidus and evaluation of sensory function or peripheral vasculature. Ulcer may extend to the bone and may be discerned by radiographic imaging or cogent bone biopsy [8,9]. Biochemical or hematological parameters are usually nonspecific, and leukocytosis, elevated erythrocyte sedimentation rate (ESR) or augmented c-reactive protein (CRP) may or may not be enunciated in osteomyelitis. c-reactive protein (CRP) is concurrent to and beneficial in monitoring response to therapy in Figure 8. Hematogenous osteomyelitis of the vertebrae, clavicle or pubic bone may be detected with appropriate blood cultures [8,9]. Employment of sensitive imaging techniques such as magnetic resonance imaging (MRI) and bone scintigraphy ameliorates diagnostic precision and possible categorization of infection [8,9].Radiographic imaging is mandatory for evaluating suspected
osteomyelitis. Additionally, plain radiographs, magnetic resonance
imaging (MRI) and technetium-99 bone scintigraphy are beneficial.
Plain radiography is the initial imaging modality demonstrating
features indicative of osteomyelitis in around 2 weeks. Also,
features of bone metastasis or fractures due to osteoporosis may be
identified [7,8]. Upon plain radiography, preliminary modifications
are discerned as swelling within adjacent soft tissue and skeletal muscle along with absence or blurring of adipose tissue planes.
Adjacent joint may depict effusion [8,9]. Alterations appearing
within two weeks are comprised of regional osteopenia, periosteal
reaction, periostitis, configuration of Codman’s triangle, focal bone
or cortical lysis, endosteal scalloping, decimation of trabecular bone,
configuration of new bone and ultimately, peripheral sclerosis with
articulation of sequestrum, involucrum or cloaca [7-9]. Typically,
soft tissue swelling, osteopenia, osteolysis, bone destruction and
nonspecific periosteal reaction are observed [9].
As plain radiographs depict lytic lesions following decimation
of around 50% of bone matrix, the modality is relatively
inadequate for discerning preliminary bone disease. Imaging at
delayed stage demonstrates a prominent periosteal reaction [7-9].
Ultrasonography is a rapid, inexpensive technique for assessing
soft tissue, joints and guiding evacuation of inflammatory exudate
although the manoeuver is inadequate for evaluating osteomyelitis
as bone penetration is unsatisfactory. Also, assessment of soft
tissue abscess, foci of cellulitis, accumulated sub-periosteal
pus, joint effusion and extra-osseous component of orthopedic
implants may be possible [7-9]. Computerized tomography (CT)
is an expensive, sensitive technique for assessing integrity of bone
cortex and trabeculae, periosteal reaction, gas within intraosseous
and soft tissue, expanse of sinus tract and necrotic bone fragments.
Computerized tomography (CT) is optimal for discerning bone
perimeter or identification of sequestrum or involucrum. The
technique is recommended for determining ensuing bone
destruction and guiding accruement of incriminated bone and soft
tissue samples [7-9].
Currently, magnetic resonance imaging (MRI) depicts
enhanced sensitivity and specificity for appropriately discerning
osteomyelitis. Preliminary lesions or bone infection can be detected
within 3 days to 5 days of disease onset. However, infection induced
by orthopedic or surgical implants may not be appropriately
detected [7-9]. MRI may be normal in preliminary instances
exhibiting clinical symptoms for nearly a week. MRI is optimal in
detecting bone marrow oedema appearing in preliminary acute
osteomyelitis. Soft tissue and joint complications can be identified.
Upon MRI, T1 signal intensity is minimal to intermediate in the
centric component with reduced signal intensity of circumscribing,
oedematous bone marrow and destroyed cortical bone. T2 signal
intensity is enhanced due to centric fluid component and bone
marrow oedema [7-9]. Administration of intravenous contrast
aids distinction between phlegmon, necrotic tissue and abscess.
Post contrast imaging depicts enhancement of bone marrow,
abscess perimeter, periosteum and aggregated, adjoining soft
tissue [7-9]. Although minimally specific, nuclear imaging is a
sensitive technique for detecting preliminary bone disease and can
be beneficially adopted in instances where MRI is circumvented
due to orthopedic implants. Three phase technetium-99m bone
scan and tagged white blood cell scan are generally employed [7-
9]. Alternatively, imaging modalities such as positron emission
tomography (PET), leukocyte scintigraphy and gallium scan can
be adopted to assess osteomyelitis [7-9]. Bone scintigraphy with
technetium- 99m (Tc-99m) scan is a sensitive technique which
depicts enhanced osteoblastic activity with elevated radiotracer
uptake within the circumscribing bone [6,7]. Indium -111
labelled white blood cell [WBC] scintigraphy is recommended in
evaluation of diabetic osteomyelitis, orthopedic implants, vertebral
osteomyelitis, or ulceration in immobilized subjects with potential
osteomyelitis [6,7].
Gallium-67 scintigraphy with exposure to radioactive gallium
demonstrates enhanced isotope uptake in conditions with infection
or sterile inflammatory conditions and malignant metamorphoses
[6,7]. Positron emission computerized tomography (PET-CT)
is a contemporary, precise modality for diagnosing chronic
osteomyelitis. Bone tissue samples may be obtained percutaneously
or with open technique [6,7]. Tissue sampling is essential for
morphological confirmation of osteomyelitis, identification of
pathogens and assessing susceptibility to diverse antibiotics in
order to ensure pertinent therapy. Superficial wound culture,
needle puncture material or tissue extracted from sinus tract is
unsatisfactory for histological confirmation [6,7]. Percutaneous
tissue samples are preferably obtained through intact cutaneous
surface, prior to commencement of antibiotics with guidance
from computerized tomography (CT) or fluoroscopy. The samples
may be utilized for histological assessment, gram’s stain, culture,
and sensitivity. Open bone biopsy is recommended, in contrast to
percutaneous tissue sampling [6,7].
Therapeutic options
Osteomyelitis is appropriately treated with surgical confinement of infection and antibiotic therapy for extended duration [8,9]. Surgical debridement of infected, necrotic bone and soft tissue is required as impregnation of antibiotics into infected fluid, abscess or injured, necrotic bone is inadequate [8,9]. Osteomyelitis associated with prosthetic joints necessitates eradication of implants. However, susceptible organisms such as streptococci may be satisfactorily managed with extended antibiotic therapy with the implanted device in place. Vacuum-assisted wound closure devices may be employed for repairing enlarged, deep wounds following extensive debridement of necrotic tissue [8,9]. Control of associated comorbidities, revascularization of implicated limb and decimation of contributing host factors which impede wound healing such as consumption of tobacco, malnutrition, chronic hypoxia, immunodeficiency, chronic lymphedema, and peripheral neuropathy is necessitated prior to surgical intervention [8,9]. Prolonged antibiotic therapy is recommended contingent to culture and sensitivity or as empirical, broad-spectrum antibiotics applicable to gram-positive and gram-negative organisms, such as cloxacillin, nafcillin, vancomycin, ceftriaxone, piperacillin or tazobactam. Antibiotic levels in bone may be decimated [8,9]. Disease sites challenging to treat, as with pelvic osteomyelitis may be subjected to several months of extended antibiotic therapy. Frequently, intravenous antibiotics may be extensively employed. Accumulation of inflammatory exudate, sequestrum or involucrum can be managed with surgical drainage or debridement [8,9].
Amputation is a manoeuver applicable to instances of unsuccessful medical therapy or life-threatening infection [8,9]. Hyperbaric oxygen therapy is not generally recommended for treating osteomyelitis [8,9]. Initiation of preliminary, aggressive treatment strategies ensure a superior prognosis of acute osteomyelitis. However, reoccurrence of infection may ensue [8,9]. Complications associated with untreated or inadequately treated osteomyelitis are septic arthritis, pathological fracture, configuration of sinus tract, squamous cell carcinoma, secondary sarcoma, bone abscess, bone deformity, systemic infection, contiguous soft tissue infection and exceptionally, secondary amyloidosis [8,9].
References
- Momodu II, Saraiya V (2021) Osteomyelitis. Stat Pearls Treasure Island Florida.
- Colston J, Atkins B (2018) Bone and joint infection. Clin Med (Lond) 18(2): 150-4.
- Schmitt SK (2017) Osteomyelitis. Infect Dis Clin North Am 31(2): 325-338.
- Kremers HM, Nwojo ME (2015) Trends in the epidemiology of osteomyelitis: a population-based study- 1969 to 2009. J Bone Joint Surg Am 97(10): 837-45.
- Lam K, van Asten SA (2016) Diagnostic Accuracy of Probe to Bone to Detect Osteomyelitis in the Diabetic Foot: A Systematic Review. Clin Infect Dis. 63(7): 944-948.
- Lima AL, Oliveira PR (2014) Diretrizes Panamericanas para el Tratamiento de las Osteomyelitis Infections de Tejidos Blandos Group- Recommendations for the treatment of osteomyelitis. Braz J Infect Dis 18(5): 526-34.
- Dutra LMA, Melo MC (2019) Prognosis of the outcome of severe diabetic foot ulcers with multidisciplinary care. J Multidiscip Healthc 12: 349-359.
- Zimmerli W (2010) Clinical practice- Vertebral osteomyelitis. N Engl J Med 362(11):1022-1029.
- Hatzenbuehler J, Pulling TJ (2011) Diagnosis and management of osteomyelitis. Am Fam Physician 84(9): 1027-1033.
- Image 1 Courtesy: About Kids Health.
- Image 2 Courtesy: Orthobullets.com.
- Image 3 Courtesy: Pathology outlines.
- Image 4 Courtesy: medicine.nus.sdu.sg.com.
- Image 5 and 6 Courtesy: You tube.
- Image 7 Courtesy: Diagnostic Histopathology.
- Image 8 Courtesy: Springer link.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...