Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4544
Research Article(ISSN: 2637-4544) 
Biophysical Biomarkers of The Fertile Window in Sub-Fertile Women: Individual Approach Volume 2 - Issue 4
Murcia Lora JM*
- Functional Reproductive Medicine and Biotechnology, Independent Researcher Clinical Consulting G&E, Logroño, Spain
Received: September 12, 2018; Published: September 19, 2018
Corresponding author: José María Murcia Lora, Clinical Consulting G&E, Logroño, Spain
DOI: 10.32474/IGWHC.2018.02.000144
Abstract
Introduction: Fertility signals from the fertile window can improve the prediction, detection and diagnosis of the fertile phase in subfertile woman. The effectiveness of the use of such signs in subfertile women can help to get pregnant. This article includes subfertiles women who incorporated fertility biomarkers of cervical secretion fertility, in the range of left kurtosis of the curve of the fertile phase.
Material and Method: This report includes 30 sub-fertile women with an average age of 34.3 years (sem 0.96); range: (26-46 years). An individual approach was adopted through the evolution of biophysical parameters of viscoelasticity and transparency of cervical secretion. The progression of the biomarkers was located in the fertile window, with the purpose of improving the perception in the recognition of the evolution of the biophysical properties of the cervical mucus.
Results: Pregnancy was obtained in 33% of the cases, in which 27% (8/30) were women under 35 years of age. Pregnancy was obtained in two cases in women older than 35 years. The success rate to achieve a pregnancy was higher in the group of women under 35 years of age. Ten cases of patients with male factor were included, achieving two cases of pregnancy equivalent to 6.6% of the sample in the group of patients under 35 years of age.
Conclusion: Subfertile couples with risk factors such as age, dilation in time to look for a pregnancy, and sperm of poor quality, may be candidates to consider advancing gestational desire, or consider protocols that help to detect the fertile window through observation of the evolutionary pattern of cervical secretion.
Keywords: Fertile Window; Sub-Fertility; Sterility; Fertile Biomarkers; Cervical Secretion; Ovulation
Introduction
The American Society for Reproductive Medicine has defined the length of the fertile period when the first day oestrone-3- glucuronide (E3G) is detected in urine, to the second day after the luteinizing hormone (LH) peaks. This period usually varies between 7 days in fertile people [1]. Actually, most studies define the period of greatest fertility since six days before ovulation until one day after ovulation. The change from infertile days to the most fertile days in the follicular phase of the menstrual cycle occurs during this interval, which is determined by the gradual development of the dynamic follicular progress of the ovulation [2,3]. This interval is different among sub-fertile couples; however, a good correlation also has been observed between biomarkers, at the beginning, and the end of the fertile period in cycles of sub-fertile women with regular and irregular menstrual periods, without specific pathology [4]. The decision to detect the fertile window in subfertile patients depends on the spontaneous pregnancy possibilities proven effectiveness [5]. In sub-fertile couples, the fertile window length varies, from the first day of normal sperm-mucus interaction to the ovulation period. The objective of this cases-study focused primarily on couples in order to increase the efficiency in the estimating of the time-specific of the fertile window to help to improve pregnancy probabilities.
Materials and Methods
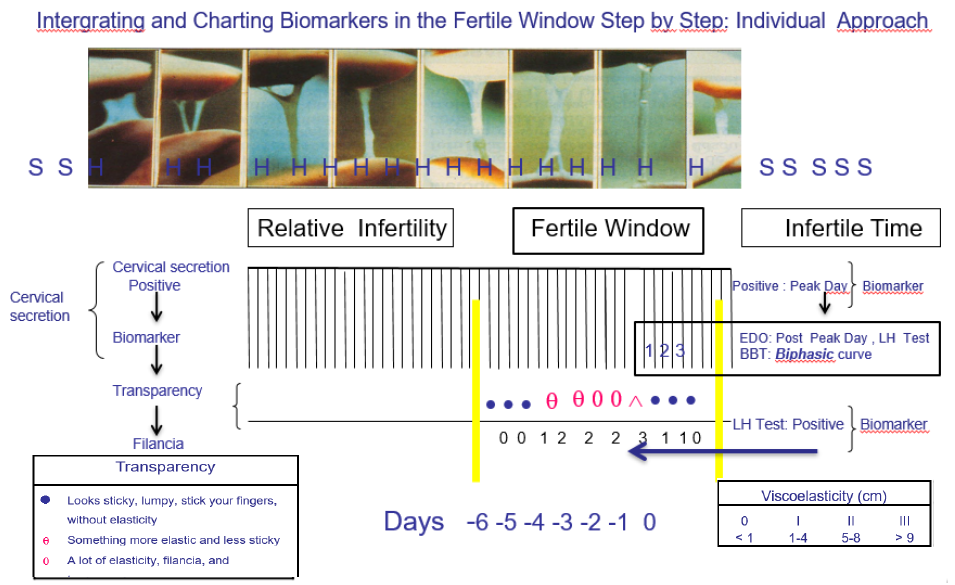
Thirty patients were included with an average age of 34.3 years (sem 0.96); range: (26 - 46 years old). This article includes subfertile women in which the left kurtosis interval of the fertile phase curve was evaluated, by checking the biophysical changing pattern of reproducible methods of viscoelasticity and transparency of the cervical secretion. The inclusion in the study take in, absence of gynecological pathology, not taking hormonal medications or drugs that affect fertility. Only subfertile couples with a reasonably good prognosis of unexplained subfertility, without any detectable cause of infertility, or if there was a cause of fertility, were corrected and incorporated into the group of subfertile women were included. The sexual relations were carried out in a fertile window. Subfertility generally describes any form of reduced fertility with a prolonged time of non-desired conception. Sterility was defined as the inability to conceive, after one year of regular intercourse without contraception. Infertility was defined as failure to achieve a live product. The individual approach was directed to the fertility markers by means of a curve plotted in which biomarkers were integrated in relation to the fertile window (Figure 1). The first day of the cycle was the first day of menstrual bleeding. “S”: Indicates the days of dryness. Days: “H” denoted humidity and the possible presence of cervical secretions for evaluation by recording two biophysical parameters: cervical viscoelasticity and transparency. The way to evaluate the elasticity was to measure the length, in centimeters (cm) of the discharge of two surfaces that are separated. As the day of maximum fertility approached, when the ovulation was close, the cervical mucus stretches more, without breaking; the amount and elasticity increase during the days before the peak day and during the same peak day. The subfertile patients recognized the evolution of the biophysical parameters of the cervical secretion when approaching the fertile window as shown in (Figure 1). The peak day was written with the following symbol “^”. The evolution of viscoelasticity was detected by a subjective measurement of the distance in cm of the cervical secretion. Viscoelasticity 0: <1cm. Viscoelasticity 1: 1-4cm. Viscoelasticity 2: 5-8cm. Viscoelasticity 3:>9cm. Transparency: ●: it looks sticky, full of protuberances, sticks fingers, without elasticity. θ: something more elastic and less sticky. 0: a lot of elasticity and transparency. The biomarkers were located within the fertility window in relation to the day of maximum fertility by means of a model photograph to facilitate the relationship of changes in cervical secretion taken by John and Evelyn Billings (Melbourne, 1972). The peak day was integrated with LH detection to explain the end of the cycle (Figure 1). We recommend 3 days after the peak day to observe a definitive change in cervical secretion. The regression of the parameters in filancia and transparency was observed, when it was not elastic, more opaque and could even disappear completely, and begin with a sensation of dryness. We provide instructions to all patients to detect the fertile window through these biomarkers and signs of fertility. We use cervical secretions, because it is well known, and can now be widely used to identify the days when there is a probability of conception in subfertile patients.
Figure 1: Photo taken by John and Evelyn Billings Melbourne 1972, and Individual Integration of biomarkers in a fertile window. Fertile window with cervical discharge: Example of -6 days before the estimated day of ovulation (EDO) up to 3 days after ovulation. The left kurtosis interval of the fertile phase curve was evaluated by controlling the biophysical change pattern of the viscoelasticity and the transparency of the cervical secretion. The objective evaluation photo helped to interpret and verify the changes in the characterization of the left kurtosis of the curve of the fertile window. “S”: indicates the days of dryness, followed by the days: “H”. “H”: denotes the humidity and the possibility of determining the cervical secretion for its evaluation, by observing two biophysical parameters of viscoelasticity and transparency.The peak day was written with the following symbol “^”.
Results
Table 1:
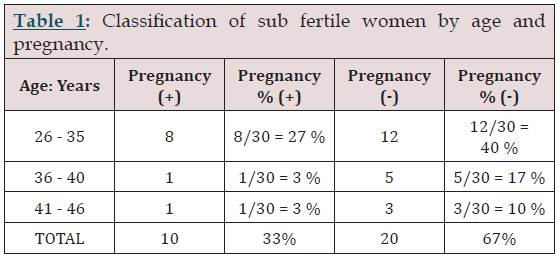
Pregnancy was obtained in 33% of the cases, of which 27%, 8/30, were in the group under 35 years of age. Pregnancy was obtained in two cases (6%) of the group of patients over 35 years old.
Table 2:
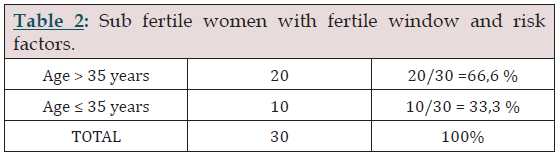
Within the factors of risk in women, age was the main risk factor that was taken into account as shown in table 2. It was possible to identify cycles and fertile window in both age groups, although success was greater to achieve one pregnancy in the group of sub fertile patients younger than 35 years.
Table 3:
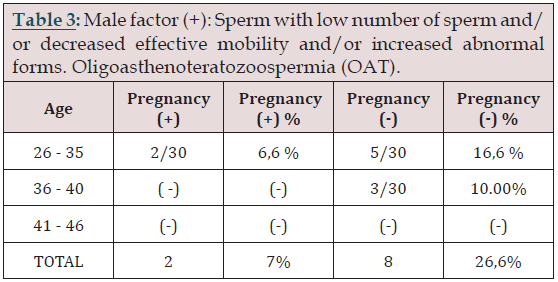
We included 10 cases of patients with oligoasthenoteratozoospermia, of which in two cases there was a pregnancy equivalent to 6.6%, of the sample in the group of patients younger than 35 years. However, patients with a male factor who wanted to attempt a pregnancy using this technology were 33%, as shown in Table 3.
Pregnancy was obtained in 33% of subfertile patients, of which 27% (8/30) were under 35 years of age. Two patients over 35 years of age remained pregnant, equivalent to 6% of the total group of elderly patients and those under 35 years of age (Table 1). Among the prognostic factors evaluated, female age was probably the most important factor (Table 2). It was possible to identify menstrual cycles and fertile window in the subfertile sub groups of patients classified by age, observing a higher success rate to achieve a pregnancy in the group of women under 35 years. We included 10 cases of patients with oligoasthenoteratozoospermia (OAT), of which a pregnancy was evidenced in two of the cases, equivalent to 6.6% of the sample in the group of patients under 35 years of age. However, a significant group of patients with male factor; 33% wanted to try a pregnancy with this methodology as shown in Table 3. The fertile period was detected by looking for biophysical parameters of the cervical secretion, in the zone of the left kurtosis of the curve of the fertile window. This was done through the evolution of the progressive changes in the secretion of the cervical mucus, from the beginning in which secretion was observed, and how it varied until the end of the fertile window. This method allowed to detect the fertile window in all patients. The main objective was to identify the biophysical changes of cervical secretion, looking for a changing pattern in the fertile window, observing the viscoelasticity and transparency. For this purpose, a tutorial of learning and follow-up was carried out through a personal interview. The age of the patients was considered adequate to consider the presence of antral follicles sensitive to a threshold of FSH, to be possible the detection of the cervical evolution in the fertile window. The typical evolution of the mucus was observed retrospectively when confirming the change of phase in the characteristics of the secretion in the fertile window, from a fertile state, to a period of infertile window. All the women had normal spontaneous menstrual cycles, in which the presence of antral follicles potentially sensitive to the threshold of FSH was documented.
The Fertile Window in Sub-Fertile Women
The term sub-fertility should be used to name any form or grade of reduced fertility in couples unsuccessfully trying to conceive [6]. Infertility (clinical definition) is currently defined as 1 year of wanted conception with unprotected intercourse in the fertile phase of the menstrual cycles [7]. There are different causes that can alter the fertility. It is basic to determine the cause of infertility; in the face of an accurate prognosis and appropriate treatment. The most frequent causes are the primary and secondary sterility causes of the female tract and the male reproduction system. A clinical diagnosis requires an organic and functional evaluation, which is based on a medical history, a physical examination, and a basic exploration by a work up of sterility, available today to the most sub-fertile women. In these subfertile patients, the causes of infertility were ruled out. Functional disorders and other causes of gynecological origin were ruled out, such as the possibility of ovarian tumors, pathology of the luteal phase or any other gynecological reason. Perhaps the irregularities in the menstrual pattern could be the most frequent situation of sub-fertility, and it could be frequent causes of stress, perimenopause, poliquistic ovary syndrome (PCOS), functional anovulatory disorders, sub clinic hypothyroidism, or sub clinic hyperprolactinemia, which can alter normal follicular development. However, any patients of these functional causes of menstrual irregularities were roulette out. Only one cause of endometriosis was considered because the patient had a medical treatment and chirurgical ovarian resection. This case become then sub-fertile and decided this option after one year without pregnancy. This case was a positive case of pregnancy. The sub-fertile patients have not different chance to conceive that to wait a spontaneous conception by looking for the best fertile window interval, when other risk factors exist. Some cumulative factors can alter fertility, among them, the most frequently cited are; advanced age, obesity, luteal phase defects, anovulatory cycles, shortening in the follicular phase, and defects in sperm capacity. Probably the major factor affecting the individual spontaneous pregnancy prospect is the time of unwanted nonconception, the age of the women [8], and the causes of sub fertility. It was eliminated; stress, psychological disturbances, and menstrual irregularities that it could interrupt the normal follicular development. It was considered one case of High Body Mass Index (BMI). Of the evaluated prognostic factors, the female age probably was the most important and limitant factor. With age, the chances of conceiving decrease, also due to the greater proportion in the heterogeneity of infertile couples. In truly sub-fertile couples, the cumulative probabilities of conception are probably independent of age, according to a recent publication in which ovulatory cycles have been achieved through controlled follicular follow-up, both in healthy patients and in sub-fertile patients with reduced follicular reserve prognostic factors [9]. A recent research established agespecific antral follicular count at different ages [10]. In general, cumulative probabilities of conception decline with age but because of increasing heterogeneity in fecundity with age, the effects mainly depend on individual factors. Therefore, the duration of unwanted non-conception remains as the main factor indicating timing of investigation in case of a subfertility problem and it mainly defines the grades of subfertility and determines prevalence estimations [11]. As expected, cumulative probabilities of conception (CPC), declined with age because heterogeneity in fecundity increases. This event was reported by the survival curves of Kaplan and Meier [12]. In general, the presented analysis takes into account the limitations of the heterogeneity of the sample, suggests that if the causes of subfertility are homogenized as previously mentioned, and the heterogeneity of the various factors affecting it is attenuated, it is possible in some cases achieve a pregnancy, even with cases of OAT and advanced age (Table 1).
Biophysical Biomarkers of Cervical Secretion in Subfertile Women
Most of the studies that compare the fertile window with traditional cervical mucus detect the fertile window with similar intervals of days. We used the cervical secretions, because it is well known, and it currently is used widely to identify the days when there is a high probability to conception in sub-fertile patients [13-15]. The changes observed in cervical mucus can be used to identify the progressive threshold of the left shift kurtosis of the fertile window of the fertile period in sub-fertile patient [16]. This method has a well-established biological justification, because estrogenic-dependent cervical mucus increases about 5–6 days prior to the day of ovulation [17,18]. There are four basically biophysical clinical parameters of cervical secretion: the volume, the viscoelasticity or spinnbarkheit of the cervical secretion, the transparency and the crystallization of cervical secretion [19]. The fertile window of ovulation using cervical secretion, was used by the World Health Organization in 1983 [20], and Hilgers’s work in 1992 [21]. A good correlation has been observed between the cervical biomarkers signals at the start and at the end of the fertile period in cycles of sub-fertile women with regular and irregular menstrual periods [22]. In normal menstrual cycles without pathology of sub-fertile women, the concentration of FSH in plasma may be the most important factor in identifying the hormonal change in sub-fertile window and in detecting the trigger points of the fertile periods. To prove this in sub-fertile patients, Kaplan-Meier charts have been showed the association between the fertile window length and the time to conception in 212 subfertile couples, in who completes fertile window length could be determined [15]. In women with normal spontaneous menstrual cycles, age-specific levels of FSH threshold level are probably the principal tool to intent this approach. It is essential that FSH levels quickly fall below the threshold even in sub-fertile women. For these sub-fertile patients, a pull of antral follicles sensitive it has been documented by ecography nevertheless de age of antral follicular cohort [10]. There is an FSH-sensitive cohort of antral follicles, which could enter in a final phase of rapid growth when the surge in FSH act, that will be stimulated, and it depends of the length of time during FSH levels above the threshold of the period when the follicles is sensitive to the influence of FSH [23]. Therefore, it was possible that a self-regulation of the hypothalamic-hypophysisgonadal- axis effectively produce an action of the FSH. This action produces the physiological changes of the cervical secretion, which could be detected. In subfertile women without pregnancy, the development of the follicular phase in the preovulatory period has been documented, with a typical increase in the cycle of the evolutionary biophysical patterns of the cervical secretion [18]. According to these results, it is possible to take into account the capacity of the follicular development, by means of an individual approach, to identify the fertile window; through observation of the vulvar or cervical mucus.
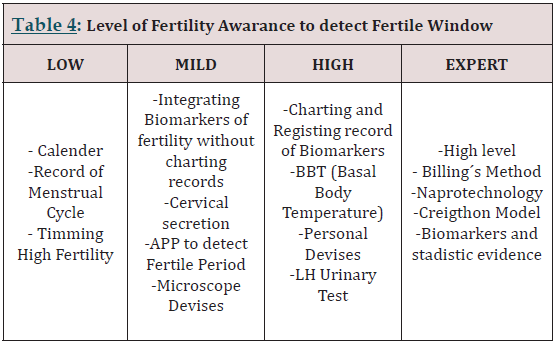
Individual Clinical Approach to Detect Fertile Windows in Subfertile Couples
An effective approach carried out as quickly as possible allows the exclusion of various causes of sterility, through a simple gynecological review with a basic study of sterility. The main objective of this article was to report some signs indicating clinical fertility biomarkers in ovulatory cycles of subfertile women. This was carried out as illustrated in Figure 1. In this way it was possible to identify the progression of the changes related to the biophysical properties of the cervical secretion, on the left side of the kurtosis of the fertile window. On the other hand, it is of general interest to mention a recent publication in which more than 3000 articles related to the fertile window and the recognition of fertility were reviewed [16]. These advances allow pregnancy with certain success in women, both in healthy populations and in subfertile women. In this article we also propose a (Table 4) with different levels of fertility perception to detect the fertile window in subfertile couples through an individual approach. It is appropriate to consider these possibilities, because there are different levels of intellectual and social culture in the awareness of the fertility approach. The adaptation and personalization of the information can help to optimize the efficacy results in subfertile couples. Four levels of counseling can be offered in individual clinical care. It could include: No experience in the use of biomarkers of fertility, previous use or non-use of the urine test to detect the fertile period. The possibility of having experience in Billings Ovulation Method, in basal body temperature, digital monitors, any other method such as Creighton Model of NaProTechnology, or biotechnological devices (Table 4). There is a good documented correlation of plasma levels of estradiol on days of high fertility in sub-fertile women by stimulated spontaneous or induced follicular cycles [24]. This event is a biomarker and a good indicator that the dominant follicle in the subfertile woman has responded to a certain threshold level of FSH. In subfertile patients, the dominant follicle and follicular maturity of the preovulatory follicle increases at a rate of approximately 1.5 to 1.7 mm per day. Follicles smaller than 9-10 mm become irregular and atresic. The increase in estrogen levels occurs in parallel with follicular growth. In turn, at the endometrial level, the influence of estrogen can be observed, such as the growth of the endometrial line and the presence of the typical three-layer echographic pattern of the periovulatory phase. Therefore, in the initial phase of the menstrual cycle, estrogen stimulates the production and release of FSH, in turn, FSH increases during the first half of the cycle. Towards the middle of the cycle, the increase in FSH and LH determines whether ovulation is possible. At the beginning of the menstrual cycle, FSH induces cell differentiation and proliferation in the preovulatory follicle. At this point in the cycle, in a sub-fertile woman a cohort of antral follicles between 2-5mm in diameter sensitive to FSH is possible [23]. When follicular stimulation occurs, increase in follicular size, a final phase of rapid growth is achieved, and a number of follicles will be stimulated, depending on the time during which the FSH levels were above the period threshold, in which the follicles were sensitive to the influence of FSH. Some follicles that have been recruited may continue to grow, despite the decrease in the concentration of FSH, but usually, only one follicle will achieve dominance. It is possible that in cases where pregnancy was not achieved, the explanation of follicular exhaustion and decreased fertility is reasonably understood by age and other factors. This analysis can help patients understand the natural limitations to seeking a pregnancy. This approach can also help to suggest that this type of methodologies are possible solutions as a scientific and therapeutic alternative in some cases.
Finally, we could say that with age, the cumulative probabilities of conception decrease, due to the heterogeneity in the factors that affect fertility, which are seen to increase in the general population due to a higher proportion of observed infertile couples. Couples with a reasonably good prognosis can be encouraged to be benefited, and candidates, for the detection of ovulatory cycles, through a followup based on follicular development, whether healthy patients or sub-fertile patients [10]. This is a representative and vulnerable sample that could be directed to higher-cost protocols. For this reason, it is prudent to develop applications and statistical models that can be applied independently in heterogeneous samples, to help the detection and optimization of fertility conditions at a given time. A novel alternative would be to develop tools with a better cost-benefit ratio.
Conclusion
The integration of some biophysical biomarkers of cervical secretion can help to better identify the changing patterns of fertility in the left area of the fertile window. In sub-fertile couples the left kurtosis curve of the fertile window may be tested using biophysical reproducible methods of fertility, by checking changing pattern at the fertile window. Sub-fertile couples with risk factors such as age, dilation in time to start a pregnancy, and sperm of low quality, may be candidates to consider advancing gestational desire over time using protocols of individual approach; such as monitoring the follicular development through the checkup of the evolutive pattern of the cervical secretion. Women who are within a subfertile population group, without an objective cause of sterility, can be candidates for personalized attention, based on individual models and strategies, which can offer some advantages and benefits in the prognosis of fertility.
References
- Fehring RJ, Schneider M (2008) Variability in the hormonally estimated fertile phase of the menstrual cycle. Fertil Steril 90 (4): 1232-1235.
- Evans-Hoeker E, Pritchard D, Long L, Herring A, Stanford J, et al. (2013) Cervical mucus monitoring prevalence and associated fecundability in women trying to conceive Fertil Steril 100(4): 1033-1038.
- Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, et al. (2000) Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod 15(12): 2478-2482.
- Ecochard R, Duterque O, Leiva R, Bouchard T, Vigil P (2015) Selfidentification of the clinical fertile window and the ovulation period. Fertil Steril 103(5): 1319-1325.
- Hilgers TW (2004) Effectiveness of NaPro technology in the treatment of infertility. In: Hilgers TW, ed. The medical and surgical practice of NaProTechnology. Omaha (NE): Pope Paul VI Institute Press, Nebraska, pp. 677-694.
- Jenkins J, Daya S, Kremer J, Balasch J, Barratt C, et al. (2004) European Classification of Infertility Taskforce (ECIT) response to Habbema ‘Towards less confusing terminology in reproductive medicine: a proposal’. Hum Reprod 19(12): 2687-2688.
- Evers JL (2002) Female subfertility. Lancet 360 (9327): 151-159.
- Hunault CC, Habbema JDF, Eijkemans MJC, Collins JA, Evers JLH, et al. (2004) Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Hum Reprod 19(9): 2019-2026.
- Steiner AZ, Pritchard D Stanczyk, FZ Kesner, JS Meadows, JW Herring, et al. (2017) Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA 318(14): 1367-1376.
- Errázuriz J, Carrasco A, Díaz E, Sanhueza P, González P, et al. (2017) Association between antral follicle count and age in fertile women Background. Rev Med Chile 145: 741-746.
- Gnoth C, Frank-Herrmann P, Freundl G, Godehardt D, Godehardt E (2003) Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod 18(9): 1959-1966.
- Kaplan EL, and Meier P (1958) Nonparametric estimation from incomplete observations. J Am Statist Assoc 53: 457-481.
- Billings EL, Billings JJ, Brown JB, Burger HG (1972) Symptoms and hormonal changes accompanying ovulation. Lancet 1(7745): 282-284.
- Hilgers TW (2004) The Medical & Surgical Practice of Na Pro technology. 1st Edn Published by: Pope Paul VI Institute Press 6901 Mercy Road Omaha, USA.
- Stanford JB, Parnell TA, Boyle PC (2008) Outcomes from treatment of infertility with Natural Procreative Technology in an Irish general practice. J Am Board Fam Med 21(5): 375-84.
- Thijssen A, Meier A, Panis K, Ombelet W (2014) Fertility Awareness- Based Method’ and subfertility: a systematic review. Facts Views Vis Obgyn 6(3): 113-123.
- Hilgers TW (2004) Effectiveness of NaPro technology in the treatment of infertility. In: Hilgers TW, ed. The medical and surgical practice of NaProTechnology. Omaha (NE) Pope Paul VI Institute Press, Nebraska, pp. 677-94.
- Fehring R (1995) A Comparison of the Peak in Cervical Mucus with the Ovule Fertility Monitor in Determining the Fertility Period. Conception Technology Inc Clinical Studies Abstract.
- Murcia JM, Esparza ML (2011) The Fertile Window and Biomarkers: A Review and Analysis of Normal Ovulation Cycles. 15(2): 133-148.
- World Health Organization (1983) Task force on methods for the determination of the fertile period: Temporal relationships between indices of fertile period. Fertile Sterile 39(5): 647-655.
- Hilgers TW, KD Daly (1992) Cumulative pregnancy rates in patients with apparently normal fertility and fertility-focused intercourse. J Reprod Med 37(10): 864-866.
- Billings EL, Billings JJ, Catarinich M (1989) Billings Atlas of the Ovulation Method. Ovulation Method Research and Reference Centre of Australia, Melbourne, Australia.
- Brown JB (1978) Pituitary control of ovarian function-concepts derived from gonadotropin therapy. Aust NZJ Obstet Gynaecol 18(1): 47-54.
- Stanford JB (2015) Revisiting the fertile window. Fertil Steril 103(5): 1152-1153.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...