
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6794
Research Article2641-6794 
Seed Bank and Seedling Recruitment Following Ten-Year Cessation of Cattle Pastures in Serrania De Los Yariguies National Park Volume 5 - Issue 4
Liliana Tinjacá Pérez and Lilia L Roa Fuentes*
- Department of Ecology, Pontificia Universidad Javeriana, USA
Received: September 16, 2020; Published: September 30, 2020
Corresponding author:Lilia L Roa-Fuentes, Department of Ecology, Pontificia Universidad Javeriana, Colombia, USA
DOI: 10.32474/OAJESS.2020.05.000224
Abstract
Seed bank and seedling recruitment following cessation of cattle pastures is a neuralgic topic to clarity the natural regeneration in a tropical forest. Land-use changes are related to the reduction in tree density and biomass, as well as changes in the vegetation distribution. A little-studied consequence is an effect on the soil seed bank and seedling recruitment, which are essential for natural forest regeneration. Natural regeneration starts the ecological succession without human intervention; otherwise, the active ecological restoration will be necessary for supporting the process. Here, we were focused on the effects of ferns and abandoned pasture vegetation on tropical rain forest regeneration. We assumed that the presence of invasive species in pastures delays the recovery time of plant communities after a disturbance. In Serranía Los Yariguíes National Park in Golconda locality, exotic pastures and ferns are the typical covers in abandoned cattle pastures. We carried out seed germination assays and field experiments involving cover clearing to evaluate the effect of soil cover on the soil seed bank and seedling recruitment. Our results showed that ferns and pastures are a factor in arresting natural forest regeneration. However, in soils with fern cover, the seed bank is a source of propagules to give continuity to the ecological succession. The creation of exclusion´s micro-sites in pasture and fern showed a significant effect on the recruitment of seedlings. The response of the soil seed bank and seedling recruitment is essential in defining the more cost-effective ecological restoration activities and support the management decisions.
Keywords: Ferns, Natural regeneration, Pasture, Secondary forest, Seed bank, Seedling recruitment
Introduction
Soil-use change to cattle pasture and agriculture are the top activities causing transformation and loss of the tropical forest [1]. The consequences of these activities include habitat loss and biodiversity reduction [2-5]. Also, the transformed areas are susceptible to invasive species’ arrival and establishment Gurrutxaga, Lozano [6], which limits the native plant community’s recovery after the human activity has ceased [1,2]. Invasive species change the soil conditions, promoting fertility loss, organic matter quantity and quality reduction, changes in the soil’s pH, and alterations in the soil’s cationic change capacity [7]. In the biological framework, invasive species reduce the soil potential for the native seed store, seed germination, and seedling establishment, with a direct effect on the native plant community’s recovery [2]. Such effects are the consequence of the high seeds production, high germination and survival rates, and high relative growth rates that define the invasive species’ behavior. Besides, invasive species display traits and biomass allocation strategies that give them an advantage in environmental adaptation over the native tree species in the absence of natural enemies. Brachiaria sp. and Urochloa decumbens (Stapf) R.D. Webster are African species typically introduced to cattle pastures [8]. What happens to such pastures after they are abandoned has attracted particular interest when it comes to an understanding of the natural regeneration processes, as well as conducting experimentation in restoration ecology and the practices of ecological restoration. Prior results have shown the extreme environmental conditions associated with high solar radiation reducing native species’ capacity to become established, as well as their rates of photosynthesis and growth [9]. Direct sun exposure increases the soil temperature and evaporation, making seeds and seedlings likelier to become dehydrated [9]. An additional stressor is the presence of ferns, especially those of the Pteridium genus, which are strong competitors in cattle pastures. Ferns are faster colonizers in areas exposed to burning, which is a common practice in cattle pasture management [9-11]. Ferns prevent the seedling establishment, deplete the soil seed bank (SB), and impede secondary succession [12].
Moreover, ferns develop a dense root layer with underground
rhizomes that have high reserves of carbohydrates and nutrients,
as well as regenerative parts that produce new fronds. The
aboveground compartment is a dense dosel, which favors shadow
and litter accumulation; the litter’s chemical composition repels
other species, thereby preventing their colonization [9,13]. In fern
areas, the remnant trees are scarce, and the natural regeneration is
reduced. However, some tree species can become established, and
previous results have shown that the litter layer favors seedlings
by replacing herbs and pastures [9].In areas with invasive plant
species, the physical and biotic characteristics of the affected
area must allow for the recruitment and germination of native
plant species to assist in the plant community’s recovery [1]. The
availability of local resources, such as soil nutrients, humidity, the
presence of microorganisms, and the availability of propagules,
are essential in the regeneration of the forest. The soil SB, defined
as all living seeds in a soil profile, including those on the soil
surface Saatkamp [14], is the central reserve of plant propagules
Chazdon,Guariguata, Martínez & Uriarte Chazdon [15], and it
determines the opportunity for natural regeneration. The SB
reduces the possibility of population extinction and promotes
species coexistence; in some cases, it is the most important source
of plants after a disturbance [16-19]. After the disturbance, the
plant regrowth is affected by propagule and resource availability
[15]. The competition with pastures and ferns and the physical
conditions can strongly affect the composition and rate of forest
regeneration [20].
The SB depends on the climate, herbivory, and disturbance. In
pastures surrounded by forest areas, a small group of plant species
present in the forest reaches the interior of the pasture, mainly
species dispersed by animals, while other species dispersed by the
wind are usually abundant. Due to the low availability of seeds in the
pastures, the recovery of tree vegetation is slow, with low diversity
earlier in succession [21]. Advanced successional forests in pastures
have small SBs (in density and composition), as the seeds of shadetolerant
species (primary, late pioneer) enter a smaller proportion
and produce a transient SB (Garwood 1989). Nonetheless, the
persistence of pastures and the presence of higher extensions of
ferns make it necessary to identify the appropriate mechanisms
that will allow the recovery of degraded areas; thereby, improving
the conservation status in Serranía de Los Yariguíes National
Park and serving as a reference to improve the connectivity with
surrounding areas. The main objective of the present study was to
determine whether the soil SB and seedling recruitment differ in
two types of soil cover in an abandoned pasture in a natural park in
northeast Colombia. Also, we hoped to determine whether a single
cutting (i.e., a single moment of pruning) of the pasture and ferns
would represent an effective strategy for accelerating the natural
regeneration processes. We expect the SB in areas with invasive
species, whether ferns or pastures, will show lower germination.
Also, we expect a simple cutting of ferns and pastures will increase
the seedling recruitment in the experimental areas.
Materials and Methods
Study area
The study was conducted in the Golconda locality (06°35’33.4” N; 73°21’15.8” W) in Serranía de Los Yariguíes National Park, which was declared a protected area in 2005 (Document N° 603, May 13). Yariguíes Park is in northeast Colombia, in the western foothills of the Eastern Andean Range, 2100 m above mean sea level. It is part of the Tropical Montane Rain Forest life zone (Holdridge 1996), and it has a mean annual precipitation of 2000 to 3000 mm in a bimodal regime, with peak levels of rainfall occurring both in April–May, and September–October. The mean annual temperature is 16.7°C [22]. Table 1 summarizes details concerning the soil characteristics across the studied sites. The study area was previously used for corn, soy, banana, and yuca (Zea maiz Vell., Glycine max (L.) Merr., Musa × paradisiaca L. and Manihot esculenta Crantz, respectively) cultivation. Posterior to cultivation the area was employed for cattle pasture; areas show loss of native vegetation and an increase of ferns (Pteridium aquilinum (L.) Kuhn and P. arachnoideum (Kaulf.) Maxon) and grasses [23]. Cattle pasture management included fire for increasing the pasture growth and herbicide application to avoid native species propagation. Forest in the study site has been affected by cattle pasture and fragmentation, with area reduction. The sites selected as reference were abandoned 60 years ago and have not been used for agriculture or grazing in recent history. The forest in the study area is a secondary forest dominated by tree species of the families Melastomataceae, Rubiaceae, Lauraceae, and Araliaceae.
Table 1: The mean and standard error of physical and chemical values of soil in the study sites into the Natural Park Serrania de Los Yariguíes, location Golconda. N = 27. **P < 0.01, ***P < 0.001. Different lowercase letters indicate means are significantly different (P < 0.05) among soil covers.
Experimental Design
To select areas, we considered the similarity in the soil use history and the time since abandonment; besides, we select sites into the same landscape matrix. We selected three soil covers, secondary tropical forest (Fo), pasture (P), and P. aquilinum (Pa). Forest is an intermedia successional forest according to local expert knowledge of specific site history. In each soil cover, we selected three sites and installed three replicated 50 m2 (10 × 5 m) plots per site. Plots in Fo were located 100 m inside from the forest border. In P, we located plots taking at least 100 m outside from the forest border. Plots in Pa were located to 100 m inside from the border of the P. aquilinum cover. In each plot, we installed eight quadrants (1 m2) for a total of 24 quadrants. In four quadrants per plot, herbaceous vegetation was cleared (i.e., herbs and shrubs in Fo, grass in P, and fern in Pa cover); the four remaining quadrants per plot were maintained with the original soil cover as control quadrants. After that, we will refer to treatments as cleared and uncleared quadrants.
Seed Bank and Seedling Recruitment
During the dry season (December 2016), we collected four soil cores from the topsoil (10 cm depth) of each plot using a soil sampler of 7.5 cm in diameter. The litter layer was removed before the soil collection. The soil samples were stored in plastic bags until the process for the seed germination assay. The samples were homogenized and distributed in a 400-cm2 plate with a perforated bottom for water drainage. We used soil for all plates to reach 2.5 cm depth, and all plates were kept in a shady greenhouse. The light and humidity conditions were similar for all plates in the greenhouse. Seedling emergence was monitored every month for six months. The seedling emergence in plates was used to estimate soil SB density and morphospecies richness. Because we were focused on the soil SB potential to recover the forest, we included tree-seedling only. In the field, we registered individual recruitment in both treatments, cleared and un-cleared quadrants, from November 2016 to August 2017. We registered seedling density (i.e., the individual number per quadrant) and morpho-species richness. To include an individual into the register, we selected this difference of grasses and ferns.
Data Analysis
The seedlings emergence in soil from the three soil covers were compared using a generalized linear model with Poisson distribution, employing the number of plants emerging in the plates. To test the effect of soil cover and treatments (i.e., cleared, or uncleared) on the seedling recruitment, we carried out a generalized linear model with Poisson distribution, the interaction between the two factors was included. To compare the rates of seed germination and plant recruitment across the three soil covers (and cleared or un-cleared treatment), an analysis of covariance (ANCOVA) was carried out. All analyses were performed using R software, version 2.15.2.
Results
Soil Seed Bank
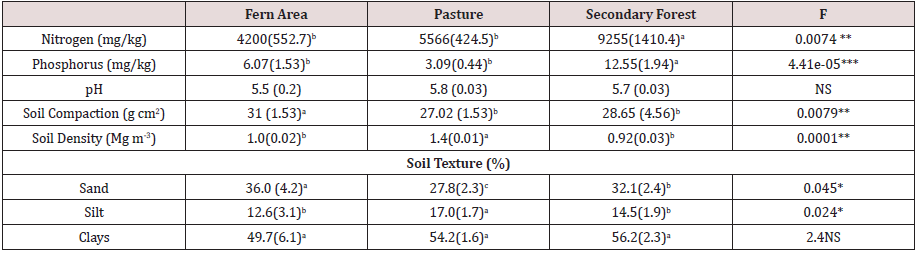
The total number of germinated seeds, considering all the soil cover plates, was 3415 in 108 soil samples. The seed germination census began 30 days after the soil preparation and continued to 194 days. We found higher seed germination in soil from P. aquilinum cover (1487 seedlings), followed by the soil from the forest (1073 seedlings) and pasture (855 seedlings). The rates of seed germination were different across the three soil covers (Z = 71.57; p = 0.001). The soil SB from the fern cover showed the highest germination rate (34.69 ± 1.20 seeds per day), followed by the soil SBs from the secondary forest (23.42 ± 1.25) and pasture (21.29 ± 1.02; Figure 1). The number of morpho-species was affected by the soil cover (Z = 14.64; p = 0.001). The richness of morpho-species was higher in P. aquilinum (4.97 ± 0.25), followed by forest (3.61 ± 0.26) and pasture (2.83 ± 0.20).
Figure 1: Germination time course and 95% confidence interval bands in the plate experiment. Soil from the forest (empty circle), P. aquilinum (gray circles), and pasture (dark circles).
Seedling Recruitment
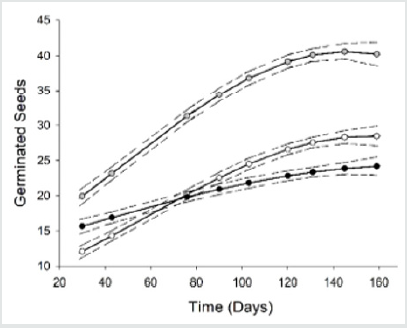
The seedling recruitment census began 57 days after the plots were installed, and it continued for 284 days. There were 16416 seedlings counted in the sampled plots across the three soil covers and two treatments. The soil cover significantly affected the number of seedlings (Z = 176.37; p = 0.0001). Most seedlings were recruited in the P. aquilinum quadrants (29.36 ± 3.06), followed by the secondary forest (17.77 ± 3.05) and pasture (17.52 ± 3.94). In the same way, the treatment affected the seedling recruitment count (Z = 72.55; p = 0.001). The greatest seedling recruitment was found in the cleared plots (19.90 ± 1.31) in comparison with un-cleared ones (12.13 ± 1.33; Figure 2). In the cleared plots, there were 10750 seedlings recruited counted, and in un-cleared plots, 6551 plants across the three soil covers. The seedling recruitment across the soil covers varied per treatment (Z = 72.55; p = 0.001). In the cleared sites, the plots in the pasture showed the biggest seedling recruitment rates (33.80 ± 2.48), followed by P. aquilinum (18.56 ± 1.95) and forest (7.35 ± 1.74). In the un-cleared plots, the secondary forest plots showed the greatest number of seedlings (18.8 ± 1.46), followed by P. aquilinum cover (14.5 ± 1.06) and pasture (13.43 ± 1.60). The morpho-species richness was affected by the soil cover and treatment (Z = 176, p = 0.0001; Z = 12.74, p = 0.001, respectively). The greatest number found was in the morphospecies number in P. aquilinum sites (4.97 ± 0.22), followed by the forest (3.58 s 0.13) and pasture (0.50 ± 0.11).
Figure 2: The mean and standard error of seedling recruited in un-cleared (dark circles) and cleared (empty circles) in secondary forest, fern lands and pasture. The error bars indicate the standard error value.
Discussion
The traditional soil used in the study sites with P. aquilinum
and pasture cover could be the factor generating the decrease of
the soil’s nitrogen and phosphorus, as well as the increase in soil
compaction beneath P. aquilinum and soil density beneath the
pasture. In contrast to our expectations, the seed density and
diversity of morpho-species in the SB from P. aquilinum areas were
higher than those found in the secondary forest. These results suggest that P. aquilinum does not limit the incorporation of seeds
into the soil, in agreement with the results reported by Xavier et
al. (2016) and Ghorbani et al. (2006). In our study area, the ferns
cover comprised of P. aquilinum and P. arachnoideum, which
have been shown to limit productive practices and biodiversity
conservation actions because they prevent seed germination
and seedling establishment and growth [11,24-26]. However, the
seedling abundance and morpho-species richness in the soil from
P. aquilinum areas allowed us to consider that the ferns were
acting in favor of the seed input and preventing seed predation
in Golconda locality. The lower seed germination in soil from the
secondary forest could have resulted from a limited seed input
of pioneer species that colonized in the early stages of secondary
succession (Guevara et al. 2005). Also, since the soil was sampled
in locations inside the forest, far away from the border, the lower
density of individual recruitment may have been a result of higher
rates of seed predation [27]. The results found in the pasture were
in concordance with our expectations and other reported findings
(Xavier et al. 2016). The low germination, as well as the lower
number of morpho-species, was evidence of the highly competitive
power of the pasture species, such as U. decumbens, limiting the
vegetation establishment and SB formation. The low seed density
in the pasture has been associated with low seed rain and high
seed and seedling predation (Esquivel et al. 2008; García-Orth &
Martínez-Ramos 2008); the additional evidence of the apparent
differences in the seedling recruitment in the presence of exotic
pastures (in the un-cleared treatment) reinforces the theory
about pasture’s invasive power. Although we did not focus on the
mechanisms for this, there is agreement on the role of pastures as
physical barriers that limit the seeds’ incorporation into the soil;
they also favor high seed and seedling predation, and they are
strong competitors for soil nutrients, water, and/or light [2,28,29].
These mechanisms may have governed the results found in the
Golconda locality. Also, although it has been reported that seed
rain in pastures declines with distance to the remnant forest, our
studied pastures were at 10–25 m from the remnant forest, thereby
falling within the limit for seed dispersion reported in the literature
(Martínez-Garza & González-Montagut 1999; Cubiña & Aide 2001).
We can expect the low seedling recruitment of the SB in the pasture
was due to seed predation; though, we need more evidence to
support this. Although our study has limited power for elucidating
the mechanisms controlling such patterns, our results are in line
with those reported on the SB dynamics in tropical forests. Our
results highlight that actions favoring natural regeneration in
pasture areas will require the enrichment of the SB.
The experimental site with a single cutting of exotic vegetation
showed this was an effective treatment. The results indicated
that, in the tropical rainforest of the National Park Serranía de Los
Yariguies, pasture and P. aquilinum control represents a useful
strategy for the development of a plant community. We also showed
the high potential for natural regeneration of fern cover, despite its
high invasive power. Most emergence of seedlings in sites where
ferns and pasture were cut once and germination assays in soil from
ferns show us that fern areas represent a considerable contribution
to the natural regeneration process in the transformed forest. In the
field, the creation of exclusion micro-sites is stimulating the seedling
recruitment, which contributes to the recovery of the diversity and
species richness in concordance with the pattern reported in the
literature [30,31]. Finally, we considered low seedling emergence
in cleared tropical secondary forest as a consequence of slow
dynamics, associated with low light availability, and possibly, an
inhibition effect by species present in the SB. In conclusion, ten
years after the land was abandoned, the effect of the productive
activities carried out in the Golconda locality has been shown and
continued to diminish the potential of the forest regeneration in
favor of the presence of invasive species. Also, we can affirm that it
is necessary to implement different management actions to achieve
forest restoration. In fern areas, although ferns are challenging
to eradicate, it is possible to take advantage of the availability of
propagules to implement active restoration actions. In the meantime,
it is necessary to implement active restoration strategies, such as
the planting of vegetation nuclei with species native to the area.
With this study, we can suggest the elimination of vegetation cover
is a practice that can facilitate natural regeneration, especially for
ferns, where there was a higher quantity of proposals and a greater
diversity of species after clearing. Finally, there is an obvious need
to carry out further work to ascertain the mechanisms governing
the plant community’s development in pasture and fern areas. The
question is vital in protected areas where there is the potential
to reverse the effect of the historical damage to the native plant
community [32-34].
Acknowledgment
We would like to thank the Serranía de los Yariguíes National Park team for the access to field sites and for their help in the field. The first author thanks Maestría en Conservación y Uso de Biodiversidad of Pontificia Universidad Javeriana for the academic and logistic support. This study was supported by Consejo Profesional de Biologia grants.
References
- Martins M, Engel V (2007) Soil seed banks in tropical forest fragments with different disturbance histories in southeastern Brazil. Ecological Engineering 31(3): 165-174.
- Meli P (2003) Restauración Ecológica de bosques tropicales. Veinte años de investigación acadé Interciencia 28: 581-589.
- González Dezzeo AN (2011) Efecto del cambio de bosque a pastizal sobre las características de algunos suelos en los llanos occidentales de Venezuela. Interciencia 36: 135-141.
- Gibson LT, Ming L, Pin B, Brook T, Gardner J, et al. (2011) Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478: 378-383.
- Vargas O (2011) Restauración ecológica: diversidad y conservació Acta Biológica Colombiana 16(2): 221-246.
- Gurrutxaga Lozano (2006) Efectos de la fragmentación de hábitats y pérdida de conectividad ecológica dentro de la dinámica territorial. Polí Revista de Geografía 16: 35-54.
- Fleischner T (1994) Ecological costs of livestock grazing in western North America. Conservation Biology 8(3): 629-644.
- Morrone O, Zuloaga F (1992) Revisión de las especies sudamericanas nativas e introducidas de los géneros Brachiaria y Urochloa (Poaceae: Panicoideae: Paniceae). Darwiniana 31 (I-4): 43-109.
- Gallegos S, Hensen I, Saavedra F, Schleuning M (2015) Bracken fern facilitates tree seedling recruitment in tropical fire-degraded habitats. Forest Ecology and Management 337(1): 135-143.
- Roos K, Rödel H, Beck E (2010) Short- and long-term effects or weed control on pastures infested with Pteridium arachnoideum and an attempt to regenerate abandoned pastures in South Ecuador. Weed Research 51: 165-176.
- Palomeque X, Günter S, Siddons D, Hildebrandt P, Stimm B, et al. (2017) Natural or assisted succession as an approach of forest recovery on abandoned lands with different land-use history in the Andes of Southern Ecuador. New Forest 48: 643-662.
- Schneider L, Fernando DN (2009) An Untidy Cover: Invasion of Bracken Fern in the Shifting Cultivation Systems of Southern Yucatán Mexico. Biotropica 42:41-48.
- Marrs R, Le Duc M, Mitchell R, Goddard D, Peterson S, et al. (2000) The Ecology of Bracken: Its Role in Succession and Implications for Control. Annals of Botany 85(B): 3-15.
- Saatkamp A Poschlod P Venable DL (2014) The Functional Role of Soil Seed Banks in Natural Communities. In: Seeds: The Ecology of Regeneration in Plant Communities 3rd Edition p-263-295.
- Chazdon R, Guariguata M (2016) Natural regeneration as a tool for large-scale forest restoration in the tropics: prospects and challenges. Biotropica 48(6): 716-730.
- Fisher J, Loneragan W, Dixon K, Veneklaas E (2009) Soil seed bank compositional change constrains biodiversity in an invaded species-rich woodland. Biological Conservation 142(2): 256-269.
- Clemente A, Rego F, Correia O (2007) Seed bank dynamics of two obligate seeders Cistus monspeliensis and Rosmarinus officinalis in relation to time since fire. Plant Ecology 190: 175-188.
- De Souza M, Leal C, Bonilha I, Overbeck G (2015) The seed bank of subtropical grasslands with contrasting land-use history in southern Brazil. Acta Botanica Brasilica 29(4): 543-552.
- Fourie S (2008) Composition of the soil seed bank in alien-invaded grassy fynbos: Potential for recovery after clearing. South African Journal of Botany 74(3): 445-453.
- Zimmerman J, Pascarella J, Aide T (2000) Barriers to forest regeneration in an abandoned pasture in Puerto Rico. Restoration Ecology 8(4):350-360.
- Bedoya J, Estévez J, Castaño G (2010) Banco de semillas del suelo y su papel en la recuperación de los bosques tropicales. Boletín cientí Centro de Museos. Museo de Historia Natural 14(2): 77-91.
- Bernal J (2011) Diagnóstico del sistema hidrológico en el Parque Nacional Natural Serranía de Los Yariguí Sistema de Parques Nacionales Naturales. Dirección Territorial Andes Nororientales.
- Morales M, Prado L, Alvarado V, Sánchez E, Díaz C, et al. (2015) Diseños para la restauración ecológica de áreas afectadas por sistemas agropecuarios en el PNN Serranía de los Yariguíes, Universidad Pedagógica y Tecnológica de Colombia, USA.
- Douterlungne D, Tacher S, Golicher D, Román F (2010) Applying Indigenous Knowledge to the Restoration of Degraded Tropical Rain Forest Clearings Dominated by Bracken Fern. Restoration Ecology 18 (3): 322-329.
- Suazo-Ortuño I, Lopez-Toledo L, Alvarado-Díaz J, Martinez-Ramos M (2015) Dynamics Soil Type and Species Forming Mono-dominant Patches: The Case of Pteridium aquilinum in a Neotropical Rain Forest Region. Biotropica 7: 18–26.
- Cunha J, Martins S, Brandão I, Lopes W (2016) Ecological restauration in area dominated by Pteridium aquilinum (L.) Kuhn in Caparaó National Park MG. Revista Árvore 41(1): e410104.
- Suzán-Azpiri H, Ponce-González O, Maldabarrera X, Cambrón-Sandoval V, Carrillo-Angeles I (2017) Edge effect on the population structure and the reproductive success of two Bursera species. Botanical Science 95 (1): 9-22.
- Holl K (1999) Factors limiting tropical rain forest regeneration in the abandoned pasture: seed rain seed germination microclimate and soil. Biotropica 31(2): 229-242.
- De Souza E, Scariot A (2014) Direct seeding of dry forest tree species in abandoned pastures: effects of grass canopy and seed burial on germination. The Ecological Society of Japan 29: 473-482.
- Raich J, Khoon G (1990) Effects of canopy openings on tree seed germination in the Malaysian dipterocarp forest. Journal of Tropical Ecology 6: 203-217.
- Khurana E, Singh J (2001) Ecology of tree seed and seedlings: implications for tropical forest conservation and restoration. Current Science 80(6): 748-757.
- Chen H, Cao MY, Tang (2013) Soil seed banks in plantations and tropical seasonal rainforest of Xishuangbanna South-west China. Journal of Tropical Forest Science 25(3): 375-386.
- Line R (2008) Germinable soil seed banks in abandoned grasslands in central and western Norway and their significance for restoration. Applied Vegetation Science 11: 223-230.
- Rubio A, Racelis A, Vaughan T, Goolsby J (2014) Riparian Soil Seed Banks and the Potential for Passive Restoration of Giant Reed Infested Areas in Webb County, Texas. Ecological Restoration 32: 4.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




