Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6794
Mini Review2641-6794 
Nitrogen Mineralization Rates in Long-Term Manured Fields Estimated using Lab Incubation Experiment Volume 4 - Issue 2
Haiying Tao*, William L Pan and Eric Bietila
Department of Crop and Soil Sciences, Washington State University, Pullman, WA, USA
Received: November 19, 2019; Published: December 02, 2019
Corresponding author: Haiying Tao, Department of Crop and Soil Sciences, Washington State University, Pullman,WA,USA
DOI: 10.32474/OAJESS.2019.04.000184
Abstract
Land application of dairy manure is a typical method for dairy farmers to recycle manure nutrients. Long-term dairy manure applications can improve soil organic matter and, as a result, potentially increase soil nitrogen availability through mineralization. However, little is known about N mineralization rates in long-term manured soils; therefore, the amount of N available to crops in any given growing season is uncertain. This study conducted a 77-day lab incubation study to estimate N mineralization rates using 102 intact cores collected from the 0 to 30.5 cm surface soils of 24 long-term manured corn-alfalfa-alfalfa-alfalfa fields and 10 nearby uncultivated native sites in Yakama County, Washington. We found that the average N mineralization rate in the longterm manured fields was 55 kg N ha-1 for every 1% SOM during the 77 days incubation period, which was much higher than the commonly used book value of 22 kg N ha-1 annually for every 1% SOM.
Keywords: Nitrogen Mineralization; Dairy Manure; Incubation; Soil Nitrogen
Introduction
Land application of dairy manure is a common practice in
confined dairy operations in the Pacific Northwest. The strategy
for nitrogen (N)-based land application of manure is to match the
manure rate to the amount of N removed by crops. This approach
calculates a recommended manure rate based on the yield goal
method for estimating crop demand [1], with adjustments for soil
test N results and soil organic matter (SOM) mineralization rate
estimates. Repeated annual N-based manure applications can
result in an accumulation of SOM because only a proportion of the
organic matter in newly applied manure is readily mineralizable for
the current growing season. The remaining organic matter can take
years to mineralize into plant available N [2].
Consequently, fields with a long history of manure applications
have the potential to experience relatively high soil N mineralization
rates during the crop growing season [3-5]. Previous studies
suggest that the N mineralization rates could be much higher than
the typical book value used in most N management guidelines
[3,4,6]. Thus, the existing management guidelines may significantly
underestimate N mineralization potential in long-term manured
fields and cause farmers to inadvertently over-apply N. Overapplication
of N reduces net profits and increases risk for N losses
to the environment.
We designed a research study to help understand the potential
N mineralization rates in long-term manured fields and reduce
some of the uncertainty surrounding N availability under these
soil conditions. The specific objectives of this study were to (1)
use a laboratory-based incubation method to estimate soil N
mineralization rates in farm fields with a history of long-term dairy
manure applications, and (2) compare these soil N mineralization
rates with those in nearby native, uncultivated soils and with
the standard book value suggested in current N management
guidelines.
Materials and Methods
Site description, intact soil core collection, and soil sampling
From dairy farms in Yakima County, WA, we selected 24 fields with a long-term (> 10 years) manure application history and 10 uncultivated, native sites adjacent to 10 of the 24 fields. We found no uncultivated sites adjacent to the other 14 fields. The average annual rainfall in the sampling areas is 229-254 mm. Soil types are sandy loam and loam. All farm fields were irrigated, alfalfaalfalfa- alfalfa-corn rotations, and managed using no-till practices. Intact soil cores were taken in May of 2016. Three intact soil cores were collected from each of the 24 farm fields and the 10 adjacent uncultivated native sites, for a total of 102 cores. Each intact soil core was sampled to 30.5 cm depth using a 10.2 cm diameter probe (Giddings Machine Company, Windsor, CO). Near each intact core sampling location, a composite soil sample comprised of three sub-samples from the surface 0-30.5 cm depth, was collected for testing soil texture and chemical properties. A certified commercial laboratory analyzed these soil samples.
Laboratory incubation
We used an aerobic incubation method for the laboratory incubation process to estimate the N mineralization rates in the field [7]. The intact soil cores were thoroughly watered to leach nitrate until no nitrate-N was detected in the leachate prior to the incubation. Soil cores were incubated at room temperature (25 °C) for 77 days from June 15 to September 3 in Washington State University’s Soil Fertility Laboratory (Figure 1). The incubation period coincided with the irrigation start and end dates in the farm fields. The soil core irrigation schedule followed the irrigation schedule in the farm fields, which was every 36 hours and less frequently toward the end of the growing season.
The leachate was collected and stored in a freezer after each irrigation. At the end of the incubation period, the samples were consolidated so that one composite sample was tested for approximately every 2 weeks. The leachate nitrate-N (NO3--N) and ammonium-N (NH4+-N) concentrations were tested using KCl extraction method. The leachate volume was recorded for each consolidated sample.
Statistical analysis
The paired comparisons between the long-term manured fields and their nearby uncultivated sites for total SOM and total N were conducted using PROC TTEST procedure [8]. The bar chart for field-by-field comparison between the long-term manured fields and their nearby uncultivated sites for SOM was graphed in Microsoft Excel [9]. The cumulative N mineralization rate for every 1% SOM and cumulative N mineralization rate collected from each soil column given the total SOM in the soil column were illustrated using scatter plot with standard error as error bar in Microsoft Excel.
Results and Discussion
Soil organic matter, total nitrogen, and inorganic nitrogen
The SOM content in 24 alfalfa/corn fields was significantly higher than the average SOM content in the 10 uncultivated sites (1.80 vs. 1.27%) (Table 1). We believe the increase in SOM in the long-term manured fields was mainly from the manure applications, as observed in long term experiments with repeated manure applications [10]. Although both alfalfa and corn crops were harvested for silage, the extended root systems of alfalfa may also have contributed to the SOM increase. Based on the composite soil sample testing, six of the 10 long-term manured farm fields had higher SOM content compared to their adjacent uncultivated sites. The other four farm fields had lower SOM content than their uncultivated counterparts, likely due to manure piled on the uncultivated sites in the past (Figure 2).
Figure 2: Soil Organic Matter content in Long-Term Manured Fields and Their Adjacent Uncultivated Locations.
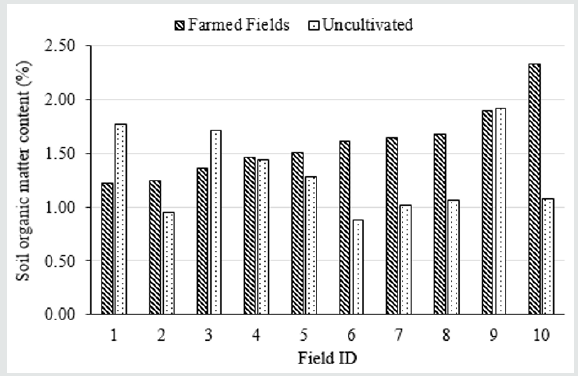
Note: p value of < .05 suggests a statistically significant difference between the long-term manured cultivated fields and native soils. Soil inorganic N included NO3--N and NH4+-N.
At the time of the composite soil sampling, the long-term manured cultivated fields had significantly higher amounts of total N than the uncultivated native sites (4,180 vs. 3,163 kg ha-1, respectively). In contrast, we found that the uncultivated soils tested higher for inorganic N (NO3--N + NH4+-N) than the manured cultivated soils prior incubation started (124 vs. 62 kg ha-1, respectively) (Table 1). This finding implies that more organic forms of N existed in the cultivated fields prior incubation. The accumulation of NO3--N in the uncultivated soils was likely due to a combination of the limited amount of existing vegetation to take up N and the dry climatic conditions that restrict N mineralization.
Nitrogen mineralization during the incubation period
Because the NH4+-N from N mineralization is quickly converted to NO3--N under optimum conditions (25 °C, near field capacity, and near neutral pH), the amount of NH4+-N in our laboratory-based leachate from the intact soil cores was negligible. During the 77- day incubation period, the average amount of N mineralized in long-term manured soils was higher compared with the native uncultivated sites. This finding is due to the higher SOM in the longterm manured soils. However, the actual rates of N mineralization for each 1% SOM in the cultivated fields and the uncultivated native sites were nearly identical, 55 vs. 59 kg N ha-1 in long-term manured fields and native uncultivated fields, respectively (Table 1 & Figure 3). Previous studies have found that mineralization rates are low under dry, natural conditions and can result in a high percentage of labile SOM [11,12]. Thus, the high mineralization rate in our uncultivated sites was likely due to decades of labile SOM accumulation, which was readily mineralizable when we provided moisture during the incubation period.
Figure 3: Average cumulative nitrogen mineralization rate per 1% soil organic matter and average cumulative nitrogen mineralization in the soil columns collected in farm fields and uncultivated sites during 77 Days of Incubation.
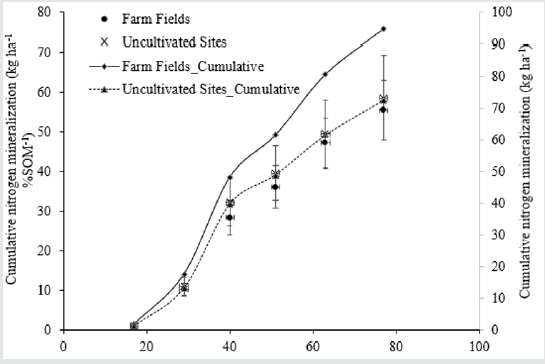
Table 1: Mean Values for Soil Chemical Parameters Prior to Incubation, and N Mineralized During the 77-day Incubation Period.
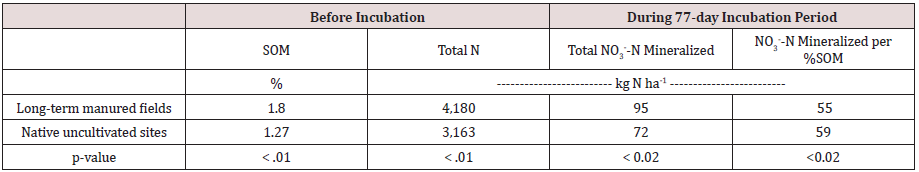
Note: p value of < .05 suggests a statistically significant difference between the long-term manured cultivated fields and native soils. Soil inorganic N included NO3--N and NH4+-N.
Importantly, our research suggests that the mineralization rates in long-term manured cultivated fields may be much higher than the typical book value of 22 kg N ha-1 year-1 for every 1% SOM [5]. Optimum soil conditions for bacterial activities are temperatures between 77-95oF and moisture levels between 50- 70% field capacity [13,14]. The lab incubation study was conducted under these optimum conditions, with a constant soil temperature of 77oF; the average daily soil temperatures in the fields during the incubation period were also mostly within the optimum range (AgWeatherNet http://weather.wsu.edu). Furthermore, because the intact soil cores were irrigated during this period of time, soil moisture was not a limiting factor for N mineralization.
However, although N mineralization is a process performed by soil bacteria, it can be affected by other environment and soil management factors that are impossible to replicate in a laboratory setting. Consequently, actual N mineralization rates under various field conditions may be different from those measured in our laboratory incubation study. Therefore, we recommend that a follow-up field study be conducted to confirm the N mineralization rates in long-term manured fields under various weather conditions.
Conclusion
Agricultural soils subject to long-term dairy manure applications had significantly higher SOM (1.80%) compared to native uncultivated soils (1.27%). We estimated N mineralization rates with a 77-day laboratory incubation study using 72 intact soil cores collected from long-term manured fields and 30 intact soil cores collected from nearby uncultivated native sites. Results showed that the N mineralization rates in these two systems were similar, likely due to decades of labile SOM accumulation in the uncultivated native sites. Importantly, we found that the rate of N mineralization in the long-term manured soils was 55 kg N ha-1 for every 1% of SOM over the 77-day incubation period, much higher than the commonly used book value of 22 kg N ha-1 year-1. Field studies of N mineralization rates are needed to verify the higher N mineralization rate in long-term manured fields.
Acknowledgement
This work was supported in part by the Washington State Department of Agriculture [Award number K1937] and the United States Department of Agriculture - National Institute of Food and Agriculture [Hatch project award number 1014527]. We also thank Dr. Linda R. Klein for valuable writing and editing assistance.
Notes
Eric Bietila, who led this research, was a research assistant in the Department of Crop and Soil Sciences, Washington State University, from 2016-2017.
References
- Tao H, Morris TF, Bravo-Ureta B, Meinert R (2016) Analyzing the implementation of nutrient management plans by farmers: implications for extension education. J Extension 54: 6.
- Herrmann AM, Witter E (2008) Predictors of gross N mineralization and immobilization during decomposition of stabilized organic matter in agricultural soil. Eur J Soil Sci 59 (4): 653-664.
- Hart JM, Sullivan DM, Myers JR, Peachey RE (2010) Sweet corn (western Oregon) nutrient management guide. EM9010-E. Oregon State University Extension Service, USA.
- Whalen JK, Chang C, Olson, BM (2001) Nitrogen and phosphorus mineralization potentials of soils receiving repeated annual cattle manure applications. Biol Fert Soils 34(5): 334-341.
- Borrelli K, Maaz T, Pan WL, Carter P, Tao H (2017) Soil Fertility Management. In: Advances in Dryland Farming in the Inland Pacific Northwest. EM108. Washington State University Extension, USA.
- Guntinas ME, Leiros MC, Trasar-Cepeda C, Gil-Sotres F (2012) Effects of moisture and temperature on net soil nitrogen mineralization: a laboratory study. Eur J Soil Biol 48: 73-80.
- Kopp K, Guillard, K. (2005) Clipping contributions to nitrate leaching from creeping bentgrass under varying irrigation and N rates. International Turfgrass Research Journal10: 79-85.
- SAS Institute (1994) The SAS system for Windows. Release 9.4. SAS Inst, Cary, NC, USA.
- Microsoft Office (2016) Microsoft Corporation. Redmond, WA, USA.
- Mulvaney RL, Khan SA, Hoeft RG, Brown HM (2001) A soil organic nitrogen fraction that reduces the need for nitrogen fertilization. Soil Sci Soc Am J 65(4): 1164-1172.
- Havlin JL, Beaton JD, Tisdale SL, Nelson WL (2005) Soil Fertility and Fertilizers. An Introduction to Nutrient Management. 7th Edition. Pearson Education, Inc. New Jersey, USA.
- Larney FJ, Buckley KE, Hao X, McCaughey WP (2006) Fresh, stockpiled and composted beef cattle feedlot manure: nutrient levels and mass balance estimates in Alberta and Manitoba. J Environ Qual 35(5): 1844-1854

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...