Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6794
Research Article2641-6794 
Evaluation the Growth Changes in Two Lettuce (Lacttuca sativa) Cultivars at Different Concentrations of Trichoderma harzianum Volume 5 - Issue 4
Shahab Ahooi1, Ladan Ajdanian1, Hossein Nemati1, Hossein Aroiee1*, Mehdi Babaei2
- 1Department of Horticultural Sciences, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran
- 2Department of Horticultural Sciences, Faculty of Agriculture and Natural Resources, University of Tehran, Tehran, Iran
Received: July 28, 2020; Published: August 18, 2020
Corresponding author: Hossein Aroiee, Department of Horticultural Sciences, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran
DOI: 10.32474/OAJESS.2020.05.000219
Abstract
In recent years, the world’s growing population and growing demand for food on the one hand, and the importance of environmental issues and sustainable agriculture on the other, have led researchers to use biomass to increase agricultural productivity per unit area. Therefore, the effect of TBI extracts from Trichoderma harzianum fungal species in increasing lettuce growth in greenhouse conditions and in the soilless cultivation system in the form of a completely randomized design with invoice arrangement was performed in 6 replications. To perform this study, 4 concentrations of 0, 5, 10, and 15% of the extract were used for each source, as well as 2 cultivars of Siaho and Gridelik. The results showed that different levels of the extract of this fungus had different effects on the growth characteristics of lettuce. Among the various levels used, the concentration level was 5% with an increase of 26.1% in a fresh weight of aerial shoot, 13.2% of the dry weight of aerial shoot, 22.29% of w fresh et root weight, 16.66% of dry root weight, 5.5 49% leaf number, 31.36% fresh leaf weight, 17.98% dry leaf weight, 20.91% leaf area, 15.78% lettuce stem diameter had the highest growth effects. Gridelik was superior in all growth factors to Siaho. Research shows that the type of plant and the type and amount of secondary metabolites secreted by different nodes and species of Trichoderma can affect the rate of their growth effects in plant interaction with Trichoderma.
Keywords: Concentration; Fungus; Soilless cultivation; Trichoderma harzianum
Introduction
Lettuce is an annual plant that has moved from coastal Europe or Central Asia to other parts of the world. However, other scholars believe that India was the origin of lettuce [1]. Lettuce has Vitamins A, B, C and other substances such as iodine, iron, phosphor, magnesium, zinc, manganese and copper in terms of nutritional value. Today, lettuce is cultivated to extract oil from its corn and consume fresh fruit [2]. Lettuce is divided into two large groups: Lactuca sativa var capitata which has two types of Butterhead and Crisphead and is produced almost in greenhouse conditions as hydroponic cultivation or soil greenhouse cultivations and Lactuca sativa var Crispa and Lactuca sativa var longifolia (which is known as Romain or Coshead) are cultivated in open space [3]. Trichoderma fungi and its different species are among the biological tools used by researchers in sustainable agriculture. Many formulations of Trichoderma have been commercially available in the market and are used as biocontrol factors against plant pathogens at small levels [4]. The antagonistic ability of different species of Trichoderma has been demonstrated against some economically important diseases. It also has the ability to occupy the supernatant and has the potential to produce extracellular enzymes and many other useful enzymes, all of which play a role in the biological inhibition of pathogens [5]. Biological inhibitors have been introduced against a wide range of pathogenic living agents such as bacteria, protozoa, nematodes and even viruses [4]. The ability of these fungal species to produce a variety of antagonistic metabolites and plant growth regulators leads to their increased biological inhibitory efficiency. The activity of Trichoderma fungi as a bio-inhibiting agent is influenced by soil physical and chemical environmental parameters [6].
Different species of Trichoderma have beneficial effects on plants. The secretion of various chemicals, high stability, and neutralization of defense compounds produced by plants and other microscopic organisms, induction of plant resistance, and defense and growth mechanisms are other factors contributing to the success of Trichoderma. It also coexists with other microscopic living organisms such as rhizobia and mycorrhizal. The ability of different extracellular enzymes in soil ، high colonization power ، root coexistence power high sporulation ability and high tolerance to the presence of field heavy metals have been recognized as a successful agent against many plant diseases [7-9]. Another advantage of Trichoderma is the improvement of photosynthesis in plants under different stresses [10]. Generally, under adverse conditions, this fungus improves plant growth and reduces stress. In addition, Trichoderma reduces production costs and adverse environmental effects [11]. Studies have shown different growth rates in plants treated with Trichoderma fungi in different hosts. On the other hand, the species of Trichoderma fungi, different strains of one species and the amount of concentration used also cause differences in the effects.
Growth enhancers of Trichoderma fungi occur on various plants. Also, the ability to enhance growth in Trichoderma depends on the type of isolate used [12]. According to available reports, the effects of plant growth-promoting by different species of Trichoderma have been proven in a number of different plants including crops, ornamental and horticultural and have been effective on greenhouse products such as beans, eggplant, lettuce, chickpeas, radishes, and peppers [13]. The inoculum of Trichoderma harzianum T22 improves growth, crop yield, leaf width and root length of maize [14]. Numerous reports on the healing effect of different species of Trichoderma harzianum.T and viride.T has been proposed in plant growth including lettuce [15,16]. Studies have shown that the production of various hormones by Trichoderma stimulates plant growth, induces growth hormones indoleacetic acid and auxin, and also improves root and shoot growth of plants [17]. Trichoderma harzianum. increases germination rate, plant height and dry weight of maize, tomato, tobacco, and radish [18]. Report on the application of harzianum. T root growth of tomato increased growth and yield of tomato, tobacco, and strawberry seedlings by producing growth-promoting compounds. Trichoderma by infiltrating the host root caused sub-root development, plant fresh weight gain, leaf width, and yield a plant [19]. Today, due to the excessive use of nitrogen-containing chemical fertilizers to accelerate vegetative growth, many vegetables, especially leafy vegetables, have a high percentage of nitrate, which in many cases exceeds established standards [20]. Lettuce is one of the leafy vegetables that have a relatively high production and consumption in Iran and is mostly consumed in fresh food. For this reason, the effects of TBI isolate from Trichoderma fungi and two cultivar on the growth factors of lettuce, including lettuce fresh and dry weight for shoot and root, stem diameter, leaf area, a number of leaves, the amount of chlorophyll and nitrate accumulation were investigated.
Materials and Method
Plant Materials and Growing Conditions
In this study, the effect of TBi isolate from a fungal species, Trichoderma harzianum, was investigated in a greenhouse experiment in a completely randomized design with a factorial arrangement with 4 replications of lettuce in Ferdowsi University of Mashhad. Four concentrations (0, 5, 10 and 15%) were considered for the fungus and two varieties of Siaho and Gridelik were also used. A system was designed with a water pump (0.5 horsepower), 9 check valves, 6 digital timers, 5 water tanks (50 liters), tape (20 cm) and required fittings and executed in re-search greenhouse of Faculty of Agriculture of the Ferdowsi University of Mashhad. This system was designed such that each row was fed only with one of the tanks. Seed of two cultivars of lettuce called Gridelik and siaho was transplanted in seedling trays and the seedlings were prepared for being transferred to the main bed after 40 days. Vases with openings of 30 ˚C were filled with 20% of cocopeat and 80% of perlite so that roots can be separated in this bed. Vases were picked in the system and seedling was transferred.
Growth of fungus in medium Davet
To prepare an extract of fungi from the Davet selective culture medium which included 1gram of nitrate calcium, 1 gram of chloride calcium, 250 mg of nitrate potassium, 250 mg of phosphate Monopotassium, 50 mg of citric acid, 2 g of sucrose, 25 g of agar, 30 mg of Streptomycin sulfate for each liter of distilled water and culture medium with 0.2 g of magnesium sulfate, 0.9 g of phosphate di-potassium, 1.5 g of potassium chloride, 3 g of glucose, 20 g of agar for each liter of distilled water were prepared [21]. This culture medium was poured in 2-liter Erlenmeyer which had been sterilized before for 20 min with an autoclave at 120 °C under the pressure of 10 atm. Now, it is time to transfer the grown biomass of fungus into these containers. In this way, a scalpel was used and pieces of the fungus with approximate dimensions of 2*2 cm along with culture medium of PDA were transferred to the containers. The containers were aerated with aquarium pipes that were connected to an air pump and kept for 8 days at 25 °C. The Erlenmeyer flasks were kept on a shaker for aeration for 8 days at 25 °C. After this period, the solid phase was isolated from the liquid phase using tiffany that fiberglass put at its bottom, and the liquid phase was kept in the refrigerator for the next steps.
Nutrition
5 sources were used, and no extract was added to source A and was regarded as control. Five volume percent of the source was added to source B, 10 volume percent of the source was added to source C and 15 volume percent of extract source was added to source D. the remaining volume of sources wad filled with Hoagland Ousley [18] nutrient solution. Source E was filled with iron and calcium and separated entered into the system. In the early days, only pure water was given to the shrubs. After ensuring full placement of shrubs in the early days, 80 ml of water and food were provided for each shrub and in the next days, they were provided considering temperature and light. PH, KOH and HCL were set about 6.5. The feeling was controlled like commercial cultivation.
Examined Traits
Fresh and dry weight (root, shoot, leaf)
The fresh weight was measured using a digital scale as 0.01 g. Next, the samples were placed in an Oven device at 74 ˚C for 4 days, the dry weight of the samples was also measured using a digital scale as 0.01 g ultimately.
Number of leaves, Leaf area and Stem diameter
The stem diameter was measured by a caliper with 0.01 mm accuracy from the upper and lower parts of the stem. Finally, the mean of these two parts was used for statistical analysis. Leaves were also counted at the end of the period and after harvest. Leaf area was measured using Leaf Area Meter (ModelLI-3100c) device and Image j software [22].
Statistical analysis
The experiment was performed in a complete randomized block design with factorial arrangement of (2×4) in six replications. To analyze the results, Minitab 16-2001 software was used. The LSD test was used for data average comparison.
Results
Fresh and dry weight (root, shoot, leaf)
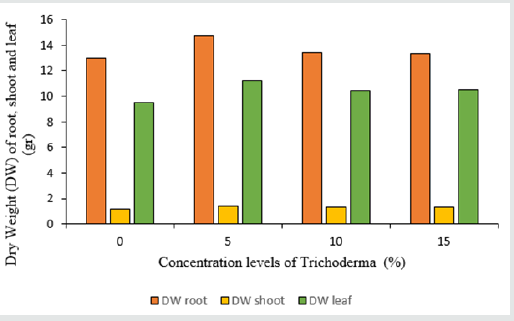
Based on the results of the analysis of variance Table 1, the application of different percentages of Trichoderma fungus had a significant effect on fresh and dry weight traits of the shoot, roots, and leaves (Table 1). The best effective concentration in fresh and dry weight of the shoot parts is 5% concentration, which is 358.8 and 14.7 gr, respectively. As the concentration used increased, there was no change in the amount of fresh and dry weight of the lettuce shoot parts (Figures 1&2) (Table 2). Also, among the different cultivars, Gridelik had the highest fresh and dry weight compared to Siaho cultivar, which increased by 24.05% and 11.8% of the fresh and dry weight of shoot organs (Table 3). The highest root weight was observed in Trichoderma treatment with a concentration of 5% to 26.4 gr (Figures 1&2), which was not significantly different from other treatments; similarly, the highest dry root weight in Trichoderma treatment was 5% to 1.4 g (Figures 1&2). There was no significant difference between 10 and 15% concentrations in dry root weight, but the lowest weight was related to control treatment (1.2 gr). Also, in related, with the cultivars used, the highest amount of fresh and dry root weight was in Gridelik cultivar compared to Siaho cultivar (Table 3). The highest fresh and dry weight of leaves were observed in Trichoderma 5% treatment at 327.2 and 11.2 gr (Figures 1&2), respectively, and the lowest rates were observed in the control treatment (249.3 and 9.5 gr), as well as Gridelik cultivar. It had the highest fresh and dry weight of the leaves compared to the Siaho variety (Table 3).
Table 1: Variation analysis of the effect of Trichoderma Bi fungal isolation at levels of 0, 5, 10 and 15% on the growth of Gridelik and Siaho lettuce cultivars.
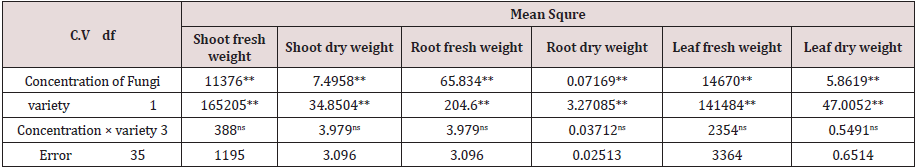
Table 2: Variation analysis of the effect of Trichoderma Bi fungal isolation at levels of 0, 5, 10 and 15% on the growth of Gridelik and Siaho lettuce cultivars.
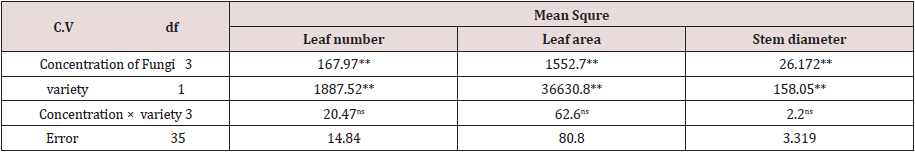
Figure 1: Comparison of the effect of Trichoderma on Fresh weight (FW) of root, shoot and leaf (p ≤ 0.05). Different letters indicate significant differences between treatments by LSD test.
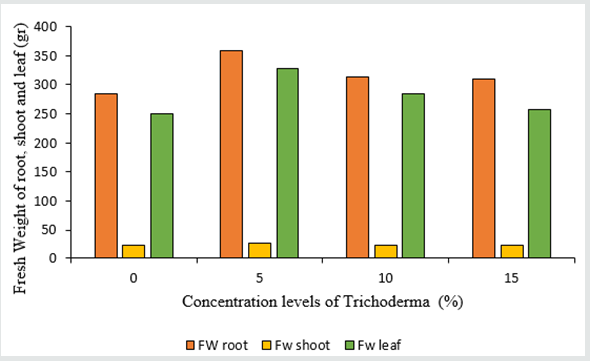
Figure 2: Comparison of the effect of Trichoderma on Dry Weight (DW) of root, shoot and leaf (p ≤ 0.05). Different letters indicate significant differences between treatments by LSD test.
Number of leaves, Leaf area and Stem diameter
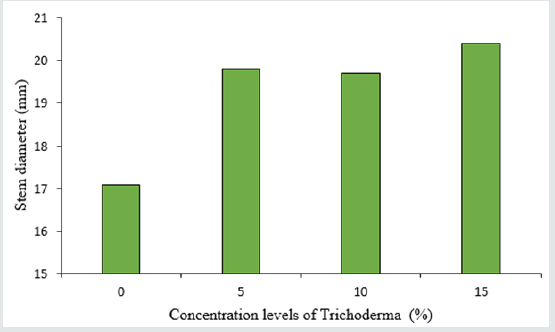
The analysis of variance table shows the significant effect of Trichoderma concentrations and different varieties of lettuce on the number and surface of leaves, as well as stem diameter (Table 2). The highest number of leaves in lettuce was 39 in the treatment of 5% Trichoderma fungus (Figure 3) and there was no significant difference between other treatments and control treatment in terms of leaf count. Among the cultivars used, the highest number of leaves was observed in the Gridelik cultivar by 37 accounts compared to the Siaho cultivar (25 accounts) (Table 3). Similarly, at the leaf area, it was observed that the treatment of 5% of Trichoderma fungi had the highest rate (238.1) and there was no significant difference between other treatments and control treatment (Figure 4). Also, the Gridelik cultivar increased by 24.33% compared to the Siaho cultivar (Table 3). The diameter of the stem in lettuce did not change significantly with different concentrations of Trichoderma fungi, but the lowest stem diameter was seen in the control treatment at 17.1 mm (Figure 5). Among the cultivars used, the highest stem diameter was seen in Gridelik treatment (21.1 mm) compared to Siaho (Table 3).
Figure 3: Comparison of the effect of Trichoderma on number of leaves (p ≤ 0.05). Different letters indicate significant differences between treatments by LSD test.
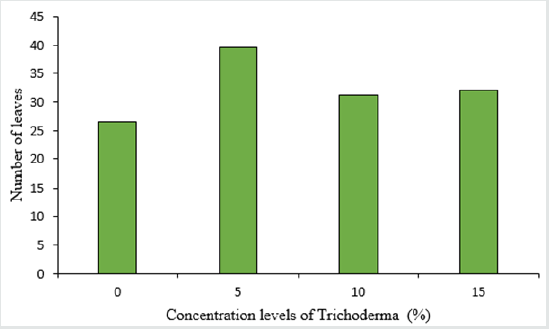
Figure 4: Comparison of the effect of Trichoderma on leaf area (p ≤ 0.05). Different letters indicate significant differences between treatments by LSD test.
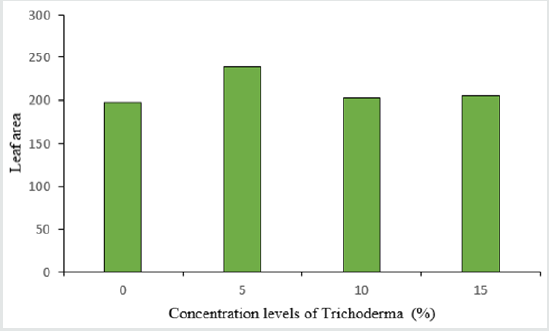
Figure 5: Comparison of the effect of Trichoderma on stem diameter (p ≤ 0.05). Different letters indicate significant differences between treatments by LSD test.
Discussion
The results of this study compare the growth effects of
Trichoderma harzianum and the use of two different varieties of
lettuce on important growth factors was including the weight of
dry shoots, roots, and leaves, as well as the number and leaf area
and stem diameter, chlorophyll content and nitrate accumulation.
Although different percentages of Trichoderma are mainly used as
antagonists against many plant pathogens, their beneficial effects
on the growth and development of many plants have been proven
[18]. The results of this study were consistent with a report that the
extract of the fungus Trichoderma harzianum isolate Bi was able to
stimulate growth in plants [18]. Yedidia [16] in their research on a
large number of plants, stated that increasing branch and leaf area
is a general role of this fungus. The complex mechanisms of this
growth increase are probably due to the production and secretion
of growth stimulant compounds into the extract of this fungus,
which also increases the efficiency of fertilizer [23]. Other studies
have significantly increased the rate at which seedlings emerge,
plant height, leaf area, and dry weight due to the inoculation of
Trichoderma fungus and its extract [24]. This fungus was proven
in two stages on lettuce, in addition to increasing growth due to
the treatment of plants by this fungus in plants such as chickpeas
Zheng and Shetty [25], Mentha arvensis Singh [26], Bean Brandão
[27], tomatoes Gravel [23] , Wheat Cavalcante [28], lettuce Bal
and Altintas [15] have also been reported. The results of this test
showed that Bi Trichoderma harzianum isolates at all levels of
concentration compared to control treatment had increasing effects
on dry and fresh weight of aerial and root organs, number of leaves,
stem diameter, fresh and dry weight of leaves. In one study, the
use of Trichoderma fungi significantly increased the fresh and dry
weight of aerial and root organs, as well as the number of leaves in
a tomato plant Newman [29], as well as the effects of this fungus on
fresh and dry root weight and shoot parts, Tobacco and tomatoes,
corn, and horseradish, were evaluated positively.
The association between Trichoderma harzianum and the
plant can increase root uptake levels, which in turn can promote
the exchange of nutrients between fungi and plants, similar to
reports of mycorrhiza coexistence with plants [30]. In addition to
the production of IAA by Trichoderma, which may increase root
volume and subsequently increase nutrient uptake. These fungi
directly increase root growth and eventually plant growth by
controlling the destructive non-pathogenic microbial population
as well as by digesting the toxic metabolites produced by this
microflora by a number of enzymes [31]. It has been proven that
one of the most important metabolites generated by T. harzianum
6-pentyl-α-pyrone, known as the plant growth stimulus at low
concentrations. This combination at higher concentrations (3-10)
caused a hindrance in the growth of wheat. The two hypotheses were
proposed that the compound functions as a semi auxin compound
(Auxin at lower concentrations cause growth in the various
organs of the plant) or are contributing to the production of auxin
production. In any case, the effect of the dose in this compound and
other similar compound in the increase or inhibition of plant growth
requires more studies [32]. On the other hand, in interpreting the
mechanism of action of plant growth stimulants, many researchers
believe that mainly different isolates of harzianum fungi stimulate
plant growth by producing biochemicals or reduce the effects of
inhibiting the growth of some compounds, existing biological and chemical toxins the soil and even changes in the number of soluble
elements [29]. The secretion of organic acids such as gluconic acid,
citric acid, and fumaric acid by Trichoderma species reduces soil pH
and ultimately increases the solubility and absorption of important
micronutrients needed for plant growth such as iron, manganese,
magnesium, mineral cations and phosphates [13].
In one study, the use of 0.1 g of Trianum commercial substance
for each tomato plant in the carnation cultivar increased the yield
by 33.34% and significantly reduced the disease of rot, which is
caused by calcium deficiency and malabsorption. Calcium uptake
is directly related to root volume and water uptake, and due to root
formation by Trichoderma and increased root volume, calcium
uptake increases and eventually disease of rot decay decreases
[33]. Due to the above explanations, the type of plant and its root
secretions, the diversity, and population of microorganisms that
stimulate plant growth and their ability to colonize around the roots
and the physical and chemical conditions of the soil, especially the
type of soil microbial flora, ultimately create complex interactions.
Any change in this interaction can change the growth conditions of
the plant [29].
Conclusion
According to various studies, Trichoderma fungus, due to its ability to colonize and spores abundantly in the soil environment, especially around the roots of most crops and non-crops, and has the ability to compete for food and high location in addition to inducing compounds that cause plant resistance Stimulating the growth of underground or shoot organs also increases the production of secondary metabolites and improves growth. The results of this study also prove the positive effects of Trichoderma fungus on improving the growth factors of lettuce. Therefore, considering the above and the results obtained in the present study, it can be hoped that in addition to the biocontrol effects of Trichoderma fungus, which is currently used in agriculture, its positive effects on increasing plant growth factors and increasing production in greenhouses can be used considered.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Highlights
a. The best cultivar used in this experiment was Gridelik, but Siaho cultivar showed higher nitrate accumulation and higher levels of chlorophyll b and a than Gridelik.
b. When the concentration of the used fungus was more than 5%, there was no significant difference between the traits, so even with a lower concentration, the best result can be obtained.
References
- Křístková E, Doležalová I, Lebeda A, Vinter V, Novotná A (2008) Description of morphological characters of lettuce (Lactuca sativa L.) genetic resources. Horticultural Science 35(3): 113-129.
- Drews M, Schonhof I, Krumbein A (1997) Content of minerals, vitamines, and sugars in iceberg lettuce (Lactuca sativa var. capitata L.) grown in the greenhouse dependent on cultivar and development stage. Gartenbauwissenschaft (Germany).
- Howell C, Stipanovic R (1984) Phytotoxicity to crop plants and herbicidal effects on weeds of viridiol produced by Gliocladium virens. Phytopathology 74: 1346-1349.
- Howell C (2003) Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant disease 87(1): 4-10.
- Chaverri P, Gazis RO, Samuels GJ (2011) Trichoderma amazonicum, a new endophytic species on Hevea brasiliensis and H. guianensis from the Amazon basin. Mycologia 103(1): 139-151.
- Etebarian H (2006) Evaluation of Trichoderma isolates for biological control of charcoal stem rot in melon caused by Macrophomina phaseolina.
- Chet I, Harman G, Baker R (1981) Trichoderma hamatum: Its hyphal interactions withRhizoctonia solani andPythium spp. Microbial Ecology 7(1): 29-38.
- Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant physiology 149(3): 1579-1592.
- Kubicek C, Mach R, Peterbauer C, Lorito M (2001) Trichoderma: from genes to biocontrol. Journal of Plant Pathology 83(2): 11-23.
- Mastouri F, Björkman T, Harman GE (2010) Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 100(11): 1213-1221.
- Poosapati S. Ravulapalli PD, Tippirishetty N, Vishwanathaswamy DK, Chunduri S (2014) Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. SpringerPlus 3: 641.
- Lynch J, Wilson K, Ousley M, Whipps J (1991) Response of lettuce to Trichoderma treatment. Letters in Applied Microbiology 12: 59-61.
- Baker R (1989) Improved Trichoderma spp. for promoting crop productivity. Trends in Biotechnology 7(2): 34-38.
- Yedidia I, Benhamou N, Chet I (1999) Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agentTrichoderma harzianum. Appl Environ Microbiol 65: 1061-1070.
- Bal U, Altintas S (2008) Effects of Trichoderma harzianum on lettuce in protected cultivation. Journal of Central European Agriculture 9: 63-70.
- Yedidia I, Srivastva AK, Kapulnik Y, Chet I (2001) Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant and soil 235: 235-242.
- Poldma P, Jaakson K, Merivee A, Albrecht A (2000) Trichoderma viride promotes growth of cucumber plants. Transactions of the Estonian Agricultural University, Agronomy 162-164.
- Ousley MA, Lynch JM, Whipps JM (1994) Potential of Trichoderma spp. as consistent plant growth stimulators. Biology and Fertility of Soils 17: 85-90.
- Harman GE (2000) Myths and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzinum T-22. Plant disease 84(4): 377-393.
- Papavizas G, Lumsden R (1982) Improved medium for isolation of Trichoderma spp. from soil [Fungi]. Plant Diseases (USA).
- Windham M, Elad Y, Baker R (1986) A mechanism for increased plant growth induced by Trichoderma spp. Phytopathology 76: 518-521.
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, and et al., (2008) Trichoderma-plant-pathogen interactions. Soil Biology and Biochemistry 40(1): 1-10.
- Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biology and Biochemistry 39(8): 1968-1977.
- Kleifeld O, Che I (1992) Trichoderma-plant interaction and its effect on increased growth response. Plant Soil 144: 267-272.
- Zheng Z, Shetty K (2000) Enhancement of pea (Pisum sativum) seedling vigour and associated phenolic content by extracts of apple pomace fermented with Trichoderma spp. Process Biochemistry 36(1-2): 79-84.
- Singh S, Tripathi A, Maji D, Awasthi A, Vajpayee P and et al., (2019) Evaluating the potential of combined inoculation of Trichoderma harzianum and Brevibacterium halotolerans for increased growth and oil yield in Mentha arvensis under greenhouse and field conditions. Industrial Crops and Products 131: 173-181.
- Brandão RS, Qualhato TF, Valdisser PAMR, de CB Côrtes MV, Vieira PM, and et al., (2019) Evaluation of Trichoderma harzianum mutant lines in the resistance induction against white mold and growth promotion of common bean. BioRxiv, 713776.
- Cavalcante RS, Lima HL, Pinto GA, Gava CA, Rodrigues S (2008) Effect of moisture on Trichoderma conidia production on corn and wheat bran by solid state fermentation. Food and Bioprocess Technology 1: 100-104.
- Newman S, Brown W, Ozbay N (2002) The effect of the Trichoderma harzianum strains on the growth of tomato seedlings, XXVI International Horticultural Congress: Managing Soil-Borne Pathogens: A Sound Rhizosphere to Improve Productivity in 635: 131-135.
- Smith DR, Doucette-Stamm LA, Deloughery C, Lee H, Dubois J, and et al., (1997) Complete genome sequence of Methanobacterium thermoautotrophicum deltaH: functional analysis and comparative genomics. Journal of bacteriology 179(22): 7135-7155.
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species-opportunistic, avirulent plant symbionts. Nature reviews microbiology 2: 43-56.
- Chang YC, Chang YC, Baker R, Kleifeld O, Chet I (1986) Increased growth of plants in the presence of the biological control agent Trichoderma harzianum. Plant disease 70: 145-148.
- Howell C, Hanson L, Stipanovic R, Puckhaber L (2000) Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathology 90(3): 248-252.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...