
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6794
Review Article2641-6794 
Suitable Catalysts for Electrosynthesis of Ammonia as Green Hydrogen Storage Volume 6 - Issue 5
Mohammed M Al Hinaai1*, Rayya Al Balushi, Mohammed Al Bahri and Thuraya Al Harthy
Department of Basic Science, A’Sharqiyah University, College of Applied and Health Sciences, Oman
Received:July 07, 2023; Published:July 19, 2023
Corresponding author:Mohammad M Al Hinaai, A’Sharqiyah University, College of Applied and Health Sciences, Department of Basic Science, Ibra, Oman, Mohammed M Al Hinaai
DOI: 10.32474/OAJESS.2023.06.000246
Abstract
Designing, synthesizing, and applying electrochemical catalysts for the generation of ammonia (NH3) from electro-reduction nitrogen gas (N2) in the presence of water (H2O) is a very attractive research topic due to the extremal trend for green hydrogen utilization in the energy production sector. It became vital to be energy-free of CO2 in order to avoid elevating the earth’s temperature and the subsequent impacts. Therefore, generating ammonia via a direct electrochemical process under ambient conditions is the most promising and scalable green hydrogen storage technique. In this review, the challenges that face commercializing experimental results are reported, and the performance of new electrochemical catalysts is described. The conclusion discusses the most important technical issues which must be considered in future research.
Introduction
Ammonia (NH3) is one of the high-potential chemical storage for green hydrogen, its usual synthesis process from nitrogen (N2) and hydrogen (H2) depends intensively on the energy from nonrenewable sources. Ammonia plays an essential role in the evolution of the human population as nitrogen-based fertilizer creates. The Haber–Bosch procedure allows the production of nearly 150 million metric tons of NH3 [1], which is responsible for consuming about 1–2% of the average global energy [2,3]. Another process is the steam-methane re-forming method which consumes about 5% of pure methane as a hydrogen source [4]. Consequently, this process alone is accountable for about 1.4% of CO2 emissions [5]. Hydrogen is considered as a future green energy resource; recently, many worldwide projects have been announced, and some of them have reached the production phase. There are many challenges and barriers in the generation of green hydrogen, including renewable electricity price, storage, and hydrogen transportation. Liquid ammonia is considered a possible high-density energy carrier (22.5 MJ kg 1), at 8 bar and 22°C. However, synthesizing ammonia with available technology is unsustainable, and new technologies must be developed to overcome environmental concerns [6]. Electrochemical synthesis of ammonia is a promising solution as it can produce green NH3 in a flexible and scalable approach to take advantage of an intermittent overflow of electricity generated by renewables [7]. Figure 1 describes the general process of electrochemical syntheses of ammonia from N2 and H2O. The electrochemical creation of ammonia at room temperature and in low-pressure situations remains unreasonable at a scalable level, and various challenges should be tackled to further empower immediate electrolytic ammonia synthesis. These challenges can be summarized as follows [8-12] (Figure 1).
Figure 1: Illustrated diagram of a solid-state H+ conducting cell where NH3 is generated from H2O (steam) and N2. Reprinted with permission [12].
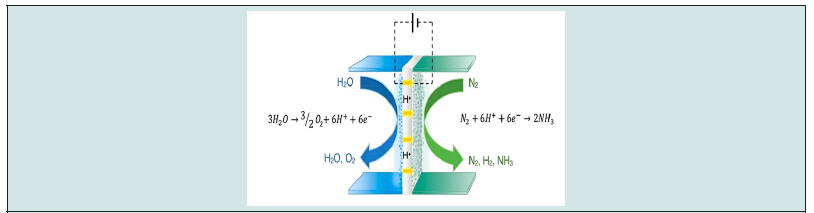
a) The reduction of nitrogen to ammonia competes with the hydrogen
evolution reaction (HER), which requires lower energy
than the nitrogen reaction because of stable tertiary covalent
bonds in (N≡N) molecules.
b) The activation energy for HER is lower due to the polar nature
of water molecules.
c) Hydrogen adsorption on the catalyst’s active sites easily occurs
at a negative potential over nitrogen adsorption. Therefore, recombining
adsorbed H on the catalyst surface is easy to generate
H2 instead of ammonia production.
d) The major issue of electrosynthesis ammonia in an aqueous
medium that the very weak solubility of nitrogen gas in an
aqueous medium.
e) Finally, most catalyst surfaces are poisoned by favorably adsorbing
oxygen traces, which deactivates active catalyst sites.
Substantial efforts have been recently made to develop new strategies to overcome the mentioned challenges in direct electrochemical nitrogen reduction to ammonia in ambient conditions. In this review, the developments and efficiency of the electrocatalysts for the synthesis of ammonia from N2, and H2O, using renewable powers will be discussed focusing on the active sites, the rate of NH3 production and faradaic efficiency (FE).
NH3 Electrocatalysts
The metallic catalysts are the most used for generating ammonia. However, heterogeneous structures are critical to enhancing the reaction rate by increasing the selectivity of the surface for NH3 generation. It has been observed that different electrolytes and electrode surface materials can lower thermodynamic conditions and enhance ammonia creation rate in several studies involving different electrolytes and electrode materials. Several compositional studies have been conducted for ammonia production with different catalysts [13]. The development of an electrochemical cell, for the electrochemical reduction of atmospheric nitrogen and water into ammonia, is a challenging task [14].
Nobel Metals catalysts
Ruthenium (Ru) based catalysts are one of the most investigated surfaces for the synthesis of ammonia and they showed higher activity than the common iron catalysts [15]. Most of these investigations were based on the usual method used for ammonia synthesis from N2 and H2 under pressure and high temperature, a view studies were done using electrochemical reduction of the N2 process in the presence of water as a source of hydrogen. In early work, an electrochemical cell for the synthesis of ammonia was developed using Ru as a cathode [16]. Only ammonia detected products from the electrochemical cell from electro- reduction of N2 at 90 °C and a potential of -1.10 V. The production rate of ammonia was very low (1.3 μg h-1m-1). The rate of ammonia generation was increased as the reduction potential value was increased until -1.02 V, and then it was decreased to a higher potential value.
Then Ru was used as single-metal sites, where it was distributed on the surface of zeolitic imidazolate framework (ZIF)-8. The formation rate of NH3 was 0.12 mg h−1mg cat −1 and Faradaic efficiency reached 29.6% when loaded mass of Ru was 0.18% [17]. N-doped porous carbon was obtained from the UIO-66 precursor to encapsulate (Ru3+ and Ru0) [18]. The single atom of Ru electrocatalyst Ru@ZrO2/NC has enough active sites to reduce N2 and produce ammonia with a rate of 3.7 mg h−1 mg−1 Ru when 0.1 wt% of the catalyst is Ru. The created catalyst exhibited high stability for 60 hours as illustrated by Figures 1 & 2. When Pt was supplemented to the Ru, the RuPt alloy improved the performance of composite (RuPt/C) which dementated 13.2% of FE at 0.123 V and generation rate of NH3 was 3.0 × 10-7 mol h−1 cm-2 [19]. Au-NPs was the matrix for developing CB (7)– K2[B12H12]@Au [20]. The ability of K+ ion in limiting and inhibiting the HER lowering the rate determining step. Therefore, the rate of ammonia formation recorded high as 41.69 μg h−1 mgcat.−1 and FE 29.53% at −0.4 V (vs. RHE).
Transition Metals catalysts
Transition metals have been investigated as active electrocatalysts. Molybdenum is another promising metal for electrochemical reduction of N2 and it forms many compounds with different nonmetallic elements. New nanodots were prepared from molybdenum carbide and then inserted in ultrathin carbon nanosheets [21]. This catalyst shows very low FE (7.8%), and the production yield was only 11.3 μg h−1 mg−1 cat. Furthermore, MoS2 was utilized as electrochemical catalyst for generation NH3 from 0.1 M Na2SO4 where generation rate recorded (8.08 × 10−11 mol s−1 cm−1) at −0.5 V (vs. RHE). The developed catalyst shows good activity even in acidic solution [22]. MoS2 nanoflowers show higher FE compared with the previous work (8.34%) and higher NH3 yield of 29.28 μg h−1 mg−1 Cat -0.40 V under same conditions [23]. MoN3 was immobilized at N-doped black phosphorus and applied to the electrochemical reduction of N2 under ambient conditions at 0.02 V [24].
The fabricated surface shown desorption free energy of NH3 is 0.56 eV, Quantum dots (QD) were prepared from black phosphorus and the MnO2 nanosheets were immobilized at the surface of QD [25]. The performance of this catalyst demonstrated a formation rate of 25.3 μg h−1 mgcat.−1 with 6.7% FE at −0.5 V (vs RHE) in 0.1 M Na2SO4 solution. Moreover, ZrO2 nanoparticles were examen for electrochemical reduction of N2 in water at ambient conditions [26]. The proposed catalyst enabled producing NH3 at a rate of 24.74 μg h−1 mg−1cat. 0.1 M HCl with 5.0% FE at -0.45 V (vs RHE). Figure 2 illustrates the effect of applied potential on the production yield of NH3 and FE using ZrO2 nanoparticles as catalysts. Likewise, a perovskite oxide of (La0.8Cs0.2Fe0.8Ni0.2O3-δ) was prepared to be applied as an electrochemical catalyst for producing ammonia from wet air [27].
Figure 2: Average NH3 yields and FEs for ZrO2/CP at each given potential in 0.1 M HCl and inset TEM image of ZrO2 nanoparticles. Reprinted with permission [26].
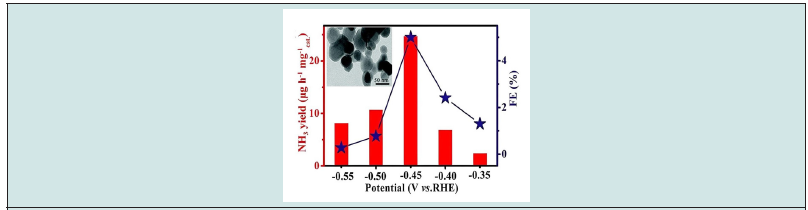
The formation rate of ammonia attained 1.23 × 10−6 mol s−1 m−2 in 400 °C cell when wet N2 (3 mol% H2O) was introduced at 1.4 V. Another, Hematite nanostructure surface was developed and used for the electrochemical synthesis of ammonia at room temperature and low pressure [28]. The generation rate of NH3 reached 0.46 μg h−1 cm−2 and FE of 6.04 % at −0.9 V (vs. Ag/AgCl) in 0.10 M KOH. The rate increased slightly after one hour and dropped by 63%.and FE was only 2.74 %. Indium-tin oxide glass (ITO/G) was used as electrochemical catalyst for formation NH3. The yield records 1.06 × 10−10 mol s−1 cm−2 and FE 6.17% at –0.40 V (vs, RHE) in 0.5 M Li- ClO4 [29] (Figure 2). Recently, the Density functional theory (DFT) calculation for the catalytic activity of Fe(100) was done to explore the ability and effect of dopant elements (Ru, Rh, Pd, Os, Ir, Pt) on the electronic properties of Fe(100) [30]. The results demonstrated that the hydrogenation step at Ir@Fe(100) was initiated at 0.342 eV with remarkably inhibited the H2 evolution.
Free metal catalysts
Synthesized black phosphorus nanosheets in zigzag and diff-zigzag edges assist selective electrochemical reduction of N2 and the greatest NH3 producing rate was 31.37 μg h-1 mg-1 cat. at -0.7 V [31]. A free metal, electrochemical carbon catalyst was doped by nitrogen, and the carbon microstructure was optimized. The developed catalyst exhibited excellent activity to the electrochemical reduction of N2, in 0.1 M KOH under ambient pressure [32]. The ammonia yield rate of 3.4 × 10−6 mol cm−2 h−1 and an FE as maximum as 10.2% at −0.3 V vs. RHE at room temperature. The formation rate reached 7.3 × 10−6 mol cm−2 h−1 when the temperature increased to 60 °C. FeN4 moiety was inserted in the carbon framework, but it showed a decrease in the ammonia production rate at same conductions. black phosphorus quantum dots (BPQDs) with the support of a conductive polymer nanofibrous membrane were tested for electrosynthesis of ammonia [33]. The yield of production in 0.1 M Na2SO4 reached 1.91 × 10−10 mol s−1 cm−2 and FE 11.9%.
Conclusion
As a conclusion, the developed electrochemical catalysts for generation of ammonia and intensive research enlighten the rode for upscale and commercialized the electrosynthesis NH3 in closed future. However, the electrochemical catalysts developed for this purpose still fall short of expectations in terms of durability and Faradaic efficiency. Also, the production rate of ammonia is still very low, and the ability to suppress hydrogen reduction utilizing the same catalyst remains a real challenge. The impressive results are recorded via free metal catalysts as they reached more than 10% FE and rate order in level 10−6 mol cm−2 h−1. This demonstrates the ability of creating catalysts from low prices precursors and safe the unabundant noble metals. Also, single atom- based catalysts improve the FE and production rate but the stability and reducing posing surface rate need more creative technic. Ru remains a desirable electrocatalyst for electrochemical generation of NH3 even its high price as it offers more than 29.6% FE and excellent production rate. More research work can be done to improve and develop electrochemical catalysts for green hydrogen storage in the form of ammonia.
References
- Soloveichik G (2019) Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process. Nat Catal 2(5): 377-380.
- Chen GF, Cao X, Wu S, Zeng X, Ding LX, et al. (2017) Ammonia Electrosynthesis with High Selectivity under Ambient Conditions via a Li+ Incorporation Strategy. J Am Chem Soc 139(29): 9771-9774.
- Smith C, Hill AK, Torrente Murciano L (2020) Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ Sci 13(7): 331-344.
- Parkinson B, Tabatabaei M, Upham DC, B Ballinger, C Greig, et al. (2018) Hydrogen production using methane: Techno-economics of decarbonizing fuels and chemicals. Int J Hydrog Energy 43(5): 2540-2555.
- Katebah M, Al-Rawashdeh M, Linke P, (2022) Analysis of hydrogen production costs in Steam- Methane Reforming considering integration with electrolysis and CO2 Clean Eng Technol 10: 100552.
- Wu T, Fan W, Zhang Y, Zhang F (2021) Electrochemical synthesis of ammonia: Progress and challenges. Mater Today Phys 16: 100310.
- Fu X, Zhang J, Kang Y (2022) Recent advances and challenges of electrochemical ammonia synthesis. Chem Catal 2(10): 2590-2613.
- Yang B, Ding W, Zhang H, Zhang S (2021) Recent progress in electrochemical synthesis of ammonia from nitrogen: strategies to improve the catalytic activity and selectivity. Energy Environ Sci 14(2): 672-687.
- Yu S, Xiang T, Alharbi NS, Al-Aidaroos BA, Chen C (2023) Recent development of catalytic strategies for sustainable ammonia production. Chin J Chem Eng.
- Amar IA, Lan R, Petit CTG, Tao S (2011) Solid-state electrochemical synthesis of ammonia: a review. J Solid State Electrochem 15(9): 1845-1860.
- Ripepi D, Zaffaroni R, Schreuders H, Boshuizen B, Mulder FM (2021) Ammonia Synthesis at Ambient Conditions via Electrochemical Atomic Hydrogen Permeation. ACS Energy Lett 6(11): 3817-3823.
- Garagounis I, Vourros A, Stoukides D, Dasopoulos D, Stoukides M (2019) Electrochemical Synthesis of Ammonia: Recent Efforts and Future Outlook. Membranes 9(9): 112.
- Abghoui Y, Skúlasson E (2015) Transition Metal Nitride Catalysts for Electrochemical Reduction of Nitrogen to Ammonia at Ambient Conditions. Int Conf Comput Sci ICCS 51(1): 1897-1906.
- Kyriakou V, Garagounis I, Vasileiou E, Vourros A, Stoukides M (2017) Progress in the Electrochemical Synthesis of Ammonia. Nitrogen Act 286: 2-13.
- Szmigiel D, Bielawa H, Kurtz M, Hinrichsen O, Muhler M, et al. (2002) The Kinetics of Ammonia Synthesis over Ruthenium- Based Catalysts: The Role of Barium and Cesium. J Catal 205(1): 205-212.
- Abghoui Y, Garden AL, Hlynsson VF, Björgvinsdóttir S, Ólafsdóttir H, et al.(2015) Enabling electrochemical reduction of nitrogen to ammonia at ambient conditions through rational catalyst design, Phys Chem Chem Phys 17(7): 4909-4918.
- Geng Z, Liu Y, Kong X, Li P, Li K, et al. (2018) Achieving a Record-High Yield Rate of 120.9 μgNH3 -1 h-1 for N2 Electrochemical Reduction over Ru Single-Atom Catalysts. Adv Mater e1803498.
- H Tao, C Choi, L X Ding, Z Jiang, Z Han, M Jia, et al. (2019) Nitrogen Fixation by Ru Single-Atom Electrocatalytic Reduction. Chem 5(1): 204-214.
- Manjunatha R, Schechter A (2018) Electrochemical synthesis of ammonia using ruthenium–platinum alloy at ambient pressure and low temperature. Electrochem Commun 90: 96-100.
- Zhao X, Yang Z, Kuklin AV, Baryshnikov GV, Ågren H, et al. (2020) Potassium ions promote electrochemical nitrogen reduction on nano-Au catalysts triggered by bifunctional boron supramolecular assembly. J Mater Chem A 8(26): 13086-13094.
- Cheng H, Ding LX, Chen GF, Zhang L, Xue J, et al. (2018) Molybdenum Carbide Nanodots Enable Efficient Electrocatalytic Nitrogen Fixation under Ambient Conditions. Adv Mater 30(46): 1803694.
- L Zhang, X Ji, X Ren, Y Ma, X Shi, et al. (2018) Electrochemical Ammonia Synthesis via Nitrogen Reduction Reaction on a MoS2 Catalyst: Theoretical and Experimental Studies. Adv Mater 30(28): 1800191.
- X Li, T Li, Y Ma, Q Wei, W Qiu, et al. (2018) Boosted Electrocatalytic N2 Reduction to NH3 by Defect-Rich MoS2 Nanoflower. Adv Energy Mater 8(30): 1801357.
- Ou P, Zhou X, Meng F, Chen C, Chen Y, et al. (2019) Single molybdenum center supported on N-doped black phosphorus as an efficient electrocatalyst for nitrogen fixation. Nanoscale 11(28): 13600-13611.
- Wang C, Gao J, Zhao JG, Yan DJ, Zhu XD (2020) Synergistically Coupling Black Phosphorus Quantum Dots with MnO2 Nanosheets for Efficient Electrochemical Nitrogen Reduction Under Ambient Conditions. Small 16(18): e1907091.
- Xu T, Ma D, Li C, Liu Q, Lu S, et al. (2020) Ambient electrochemical NH3 synthesis from N2 and water enabled by ZrO2 nanoparticles. Chem Commun 56(25): 3673-3676.
- Lan R, Alkhazmi KA, Amar IA, Tao S (2014) Synthesis of ammonia directly from wet air using new perovskite oxide La0.8Cs0.2Fe0.8Ni0.2O3-δ as catalyst. Electrochimica Acta 123: 582-587.
- Cui X, Tang C, Liu XM, Wang C, Ma W, et al. (2018) Highly Selective Electrochemical Reduction of Dinitrogen to Ammonia at Ambient Temperature and Pressure over Iron Oxide Catalysts. Chem Eur J 24(69): 18494-18501.
- Wang T, Li S, He B, Zhu X, Luo Y, et al. (2021) Commercial indium-tin oxide glass: A catalyst electrode for efficient N2 reduction at ambient conditions. Chin J Catal 42(6): 1024-1029.
- Kong H, Ma P, Zhang W, Jia M, Song W (2023) First-principles study of noble metal atom doped Fe (100) as electrocatalysts for nitrogen reduction reaction. Mater Chem Phys 297: 127396.
- Zhang L, Ding LX, GF Chen, Yang X, Wang H, (2019) Ammonia Synthesis Under Ambient Conditions: Selective Electroreduction of Dinitrogen to Ammonia on Black Phosphorus Nanosheets. Angew Chem Int Ed 58(9): 2612-2616.
- Mukherjee S, Cullen DA, Karakalos S, Liu K, Zhang H, et al. (2018) Metal-organic framework-derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes. Nano Energy 48: 217-226.
- Tang L, Dai J, Liu YT, Li ZL, Yi TF, et al. (2021) Black phosphorus quantum dots supported by a conductive polymer nanofibrous membrane: A self-standing, metal-free electrocatalyst for nitrogen fixation. Compos Commun 23: 100551.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




