Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Research Article(ISSN: 2637-4706) 
Synthesis and Antibacterial Evaluation of Some New Pyrimidine, Pyridine and Thiophene Derivatives Volume 3 - Issue 5
Abdel Haleem M Hussein*, Abu-Bakr AM El-Adasy, Ahmed AM El-Shareef, and Ahmed A Khames
- Department of Chemistry, Faculty of Science, Al-Azhar University, Egypt
Received: March 13, 2021; Published: March 23, 2021
Corresponding author: Abdel Haleem M. Hussein. Department of Chemistry, Faculty of Science, Al-Azhar University, Egypt
DOI: 10.32474/DDIPIJ.2021.03.000177
Abstract
New series of pyrimidine, pyridine and thiophene derivatives were prepared by reaction of appropriate 3-oxobutanamides with urea, thiourea, active methylene compounds, arylidines, salicyaldehyde, chalcones, benzyl isothiocyanate and aminopyrazoles. Compounds 2a, 3a, 3c and 9 show activity against some bacterial species, whereas, no activity was observed for compounds 3b, 4, 5, 7e, 7f, 10 and 11 against all bacterial species (Graphical Abstract).
Keywords: p-Aminobenzoic acid; 3-oxobutanamides; pyrimidines; pyridines; Thiophenes; antimicrobial activity
Introduction
Derivatives of p-Aminobenzoic Acid (PABA) have shown interesting pharmacological properties [1,2]. treatment of the Alzheimer’s disease [3]. it has also been used against typhus [4,5]. antimicrobial and anticancer property [2,6]. Also, many of PABA derivatives were reported for their potential inhibitory property against novel antibacterial targets –MDR-associated proteins [7,8]. antiviral targets (neuraminidase) and antifungal targets [9,10]. 3-Oxobutanamide are valuable intermediates in synthetic organic chemistry. Recently we reported a variety of synthesis of heteroaromatics that have been developed utilizing 3-oxobutanamides as readily obtainable compounds [11,12].
Results and Discussion
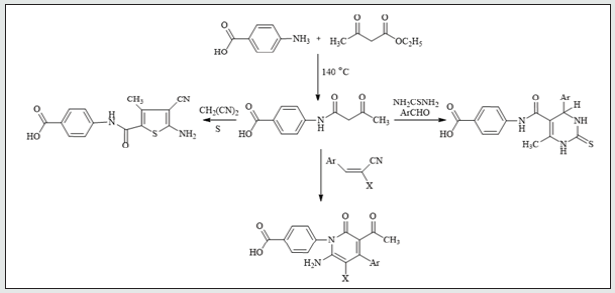
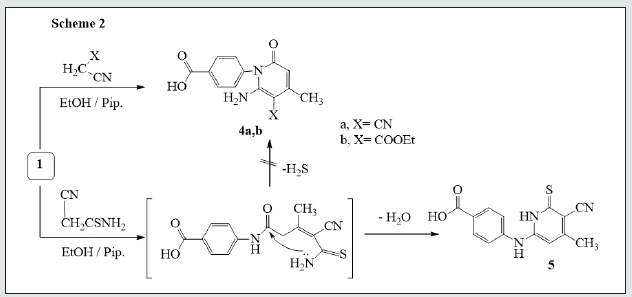
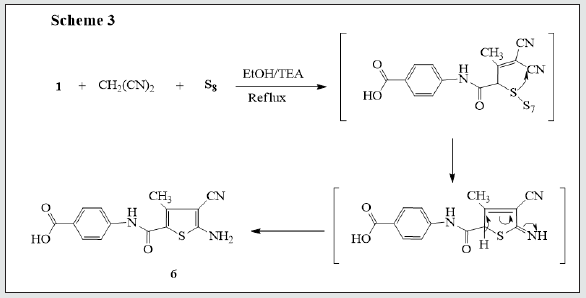
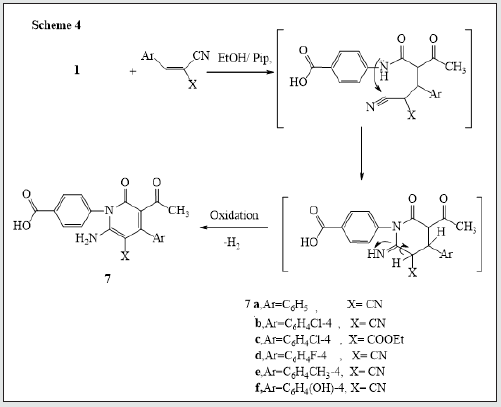
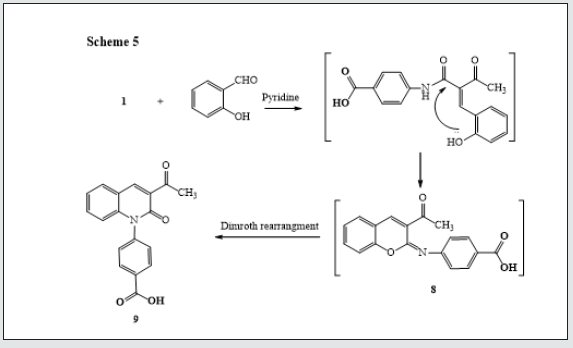
It has been found that reactions of 3-oxobutanamide [13]. 1 with some electrophilic and nucleophilic reagents to produce some new substituted azines and azoles moiety. So, treatment of 3-oxobutanamide 1 with urea or thiourea in EtOH/TEA afforded the pyrimidine derivatives 2a,b. Establishing of compounds 2a,b based on its elemental analysis and spectral data (IR, 1H NMR, 13C NMR). 1H NMR of compound 2a as example revealed a singlet signal at δ 2.00 ppm assigned to CH3, a singlet signal at δ 6.03 ppm assigned to pyrimidine-H, a multiplet signal at δ 7.59-8.00 ppm assigned to aromatic protons, 2NH appeared at δ = 9.4, 9.9 ppm and noted signal at δ = 12.83 assigned to OH. 13C NMR showed a singlet signal at δ = 18.99 assigned to CH3, a singlet signals at δ 56.51 assigned to pyrimidine-H and signals at δ 164.16, 167.33 assigned to two carbonyl group, in addition to carbon signals of aromatic structure (Figure 1). The pyrimidinediones [14,15]. 3a-c were synthesized by reaction of 3-oxobutanamide 1 with a mixture of aromatic aldehydes and thiourea. 1H NMR spectrum of 3a as example revealed the signal at δ = 2.10 ppm assigned to CH3, a singlet signal at δ 6.57 ppm assigned to pyrimidine- H, a multiplet signals at δ 7.26-8.19 ppm assigned to aromatic protons and NH group, a singlet signal at δ 9.44, 10.01 ppm assigned to two NH group, and hump at δ 11.89 ppm assigned to OH of carboxylic group. 13C showed a singlet signal at δ 17.01 assigned to CH3, a singlet signal at δ 60.91 assigned to pyrimidine-H, a signals at δ 160.08, 185.47 assigned to two carbonyl group, in addition to carbon signals in structure (Figure 1). The reaction of 3-oxobutanamide 1 with active methylene reagents was studied. So, the reaction of 1 with malononitrile, ethyl cyanoacetate in ethanolic piperidine afforded the pyridone derivatives 4a, b in good yield. 1H NMR of 4a as example revealed a singlet signal at δ 2.19 ppm assigned to CH3, a singlet signal at δ 5.68 ppm assigned to pyridine-H, a singlet signal at δ = 6. 83 ppm assigned to NH2 group and OH group noted at δ 12.90 ppm. 13C NMR of compound 4a appeared a singlet signal at δ 18.69 ppm assigned to CH3, a singlet signal at δ 69.79 ppm assigned to CH- pyridine, cyano group was detected at δ 114. 11 ppm and a singlet signals at δ 160.91, 167.54 ppm assigned to (2C=O) in addition to carbon signals in structure. Similarly, the reaction of 1 with cyanothioacetamide in ethanolic piperidine solution yield the expected pyridinethione derivative 5 under the same reaction conditions, (Figure 2). Treatment of 3-oxobutanamide 1 with malononitrile and elemental sulfur as an application of the well-known Gewald’s thiophene synthesis yield the polyfunctionally substituted thiophenebutanamide 6, (Figure 3).Treatment of compound 1 with electrophilic reagents under alkaline condition was investigated. So, the reaction of 3-oxobutanamide 1 with arylidinemalononitrile or arylidine cyanoacetate in ethanolic piperidine gave the pyridine derivatives 7. Structure 7 was confirmed as the reaction product on the basis of its elemental analysis and spectroscopic data, 1H NMR spectrum of 7c as example showed a triplet signal at δ 1.21 ppm assigned for CH3 ester, a singlet signal at δ 1.25 ppm assigned to (CH3), quartet signal at δ 4.29 ppm for CH2 ester, multiple signals at δ 7.33-8.43 ppm corresponding to aromatic protons, NH2 and hump at δ 12.92 ppm assigned to OH group. 13C NMR of compound 7c appeared a singlet signal at δ 14.16, 14.41 ppm assigned to (2CH3), singlet signal δ 62.92 ppm assigned to CH2 group, and signals at δ 154.12, 158.16, 162.09, 167.21 ppm assigned to (4C=O) in addition to aromatic carbons, (Figure 4).The quinoline derivative 9 was obtained in good yield by reaction of 3-oxobutanamide 1 with salicylaldehyde in refluxing pyridine solution through the intermediate 8 which transformed by Dimroth rearrangement [16]. To 4-(3-Acetyl-2-oxoquinolin-1(2H)-yl) benzoic acid (9). Establishing compound 9 based on the spectroscopic data. 13C NMR showed signal at δ 18.95 ppm (CH3), signals at δ 167.92, 172.05, 187.31 ppm (3C=O), in addition to carbon signals in structure, (Figure 5).
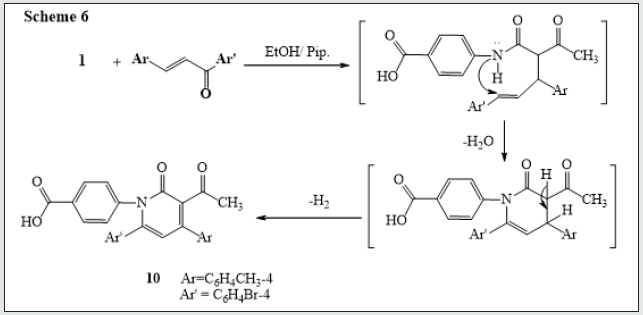
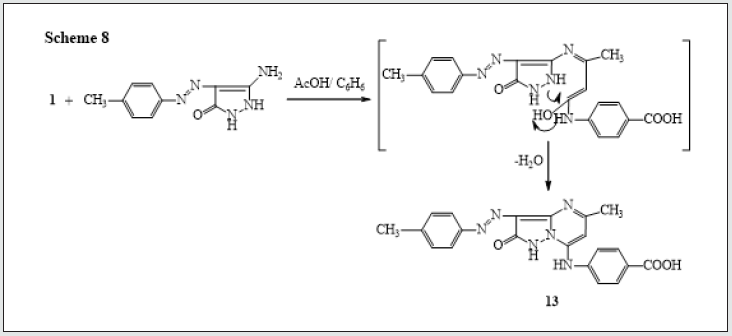
Also, reactions of 3-oxobutanamide 1 with chalcone derivative in ethanolic piperidine yield the pyridine derivative 10 [17]. via elimination of water. The 1H NMR of compound 10 revealed the presence of a singlet signals at δ 2.21, 2.36 ppm assigned to (2CH3), a multiplet signals at δ 6.62-8.11 ppm assigned to aromatic protons, and hump at δ 12.76 ppm for OH group. (Figure 6). Treatment of 3-oxobutanamide 1 with benzoyl isothiocyanate in refluxing acetone afforded the unexpected pyridine 11 rather than the expected pyrimidine 12. Structure 11 was assigned for the reaction product based on spectroscopic data (IR, 1H NMR and 13C NMR) (Figure 7). On the other hand, condensation of compound 1 with amino pyrazole derivative [18]. gave the condensation product 13 with loss of two water molecules (Figure 8).
Antibacterial activity
Evaluation of in vitro antibacterial activity of synthesized compounds was carried out using agar disc diffusion method [19]. against the growth of six pathogenic bacterial isolates; three Gram-positive bacteria (Bacillus cereus, Micrococcus luteus and Staphylococcus aureus) and three Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa and Serratia marcescens). Nutrient agar plates previously inoculated with 24 h old broth cultures of the bacterial strains were used for the antibacterial activity. Pre sterilized filter paper discs (What man No. 3, 6 mm in diameter) were loaded with 15 µl of the tested compounds (conc.10%) and allowed to dry in a laminar flow biological safety cabinet. The discs were placed aseptically on the surface of the inoculated solidified plates at equal distances. All plates inoculated with bacteria were kept in the refrigerator at 4°C for 1 h to allow for diffusion of extracts and to minimize the effects of variation in time between the applications of different solutions. The plates were incubated for 24 h at 37°C for bacteria, and then observed for the presence of inhibition of bacterial growth that was indicated by a clear zone around the discs. The diameter of the zones of inhibition (with paper discs) was measured in millimeters. Control assay discs impregnated with the antibiotic’s chloramphenicol (250 µg/ml) served as the positive controls. The antibacterial activities of the tested compounds were estimated on the growth of six pathogenic bacteria representing three Gram-positive bacteria (B. cereus, M. luteus, and S. aureus) and three Gram-negative bacteria (E. coli, P. aeruginosa and S. marcescens) by disc diffusion method. According to the results, compounds (2a, 3a, 3c and 9) showed antibacterial activity against all tested bacteria and compound 3c had the highest activity with inhibition zones ranged 13-16 mm (Table 1). Compound 7c showed a weak activity against B. cereus, E. coli and P. aeruginosa, while compounds 6 and 7b were active against P. aeruginosa and compound 7d against E. coli. On the other hand, compounds 3b, 4, 5, 7e, 7f, 10 and 11 did not exhibit any effect on the tested bacteria.
Table 1: Antibacterial activities of the investigated compounds against pathogenic bacterial isolates by disc diffusion assay.
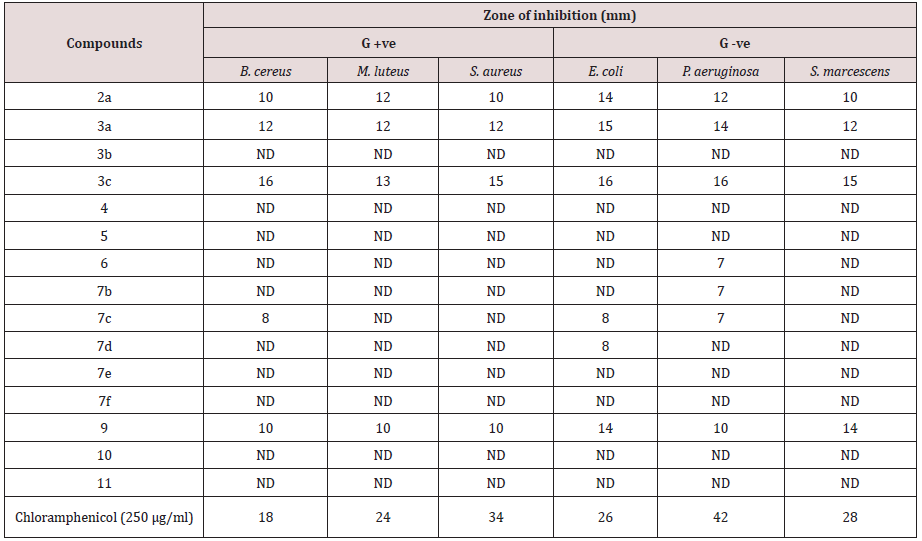
ND=Not detected
Conclusion
we have developed a simple, efficient procedure for the synthesis of some substituted pyrimidine, pyridine and thiophene derivatives was carried out using 3-oxobutanamides with some electrophilic and nucleophilic reagents, Spectroscopic data were introduced as well as reactivity indices, some of newly synthesized compounds have antibacterial activities.
Experimental
All melting points are uncorrected. IR spectra (KBr) were recorded on a FTIR 5300 spectrometer (δ, cm-1). The 1H NMR spectra were recorded in DMSO-d6 at 500 MHz on a Broker NMR spectrometer (δ, ppm) using TMS as an internal standard. Elemental analysis was carried out by the Micro analytical Research Center, Faculty of Pharmacy at Buni Swef University and Sohag University. Micro analytical Research Center, Assiut University δ δ.
General procedure for preparation of compounds (2a, b)
A mixture of 1 (0.01 mol), urea (0.01 mol) or thiourea (0.01 mol) in ethanol (30 mL) containing catalytic amount of piperidine was heated under reflux for 6 h. The separated solid product was filtrated off, washed with water and recrystallized by the proper solvent to give 2a,b.
4-((6-Methyl-2-oxo-1,2-dihydropyrimidin-4-yl) amino)benzoic acid (2a, C12H11N3O3)
Brown crystals from ethanol, Yield (62%), m.p = 220 °C, IR (KBr) ν = 3410 (OH), 3230, 3160 (2NH), 1698,1646 (2C=O) cm-1. 1H NMR (DMSO-d6) δ = 2.00 (s, 3H, CH3), 6.03 (s, 1H, CH-pyrimidine), 7.59-8.00 (m, 4H, Ar-H), 9.4,9.9 (s, 2H, 2NH) and 12.83(s, 1H, OH) ppm.13C NMR δ = 18.99 (CH3), 56.51, 117.89, 119.02, 119.46, 121.37, 126.46, 130.87, 143.20, 143.34, 164.16 (CO), 167.33 (CO). Anal. Calcd. For C12H11N3O3 (245.23): C, 58.77; H, 4.52; N, 17.13. Found: C, 58.80; H, 4.63; N, 17.25%.
4-((6-Methyl-2-thioxo-1,2-dihydropyrimidin-4-yl) amino)benzoic acid (2b, C12H11N3O2S).
Compound 2b was obtained as brown crystals from ethanol, Yield (65%), m.p = 198°C,IR (KBr) ν = 3430 (OH),3305,3260 (2NH), 1691 (C=O) cm-1.1H NMR (DMSO-d6) δ = 2.22 (s,3H,CH3), 6.53(s, 1H, CH-pyrimidine), 7.61-8.06(m, 5H, Ar-H+NH),10.56 (s, 1H, NH) and12.90 (hump, 1H,OH) ppm. Anal. Calcd. For C12H11N3O2S (261.30): C, 55.16; H, 4.24; N, 16.08; S, 12.27.Found: C, 55.27; H, 4.30; N, 16.18; S, 12.35%.
General procedure for preparation of compounds (3a-c)
To a solution of 1(0.01 mol) in ethanol (30 mL) containing hydrochloric acid (5 mL), thiourea (0.01 mol), benzaldehyde or p-methyl- benzaldehyde or p-chloro benzaldehyde (0.01 mol) were added respectively. The reaction mixture was heated under reflux for 12h, the separated solid was filtrated washed with water and recrystallized from the proper solvent to give 3a-c.
4-(6-Methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbox-amido) benzoicacid (3a, C19H17N3O3S)
Compound 3a was obtained as dark red from ethanol Yield, (67%), m.p = 118 °C. IR (KBr) n = 3470 (OH), 3324, 3309, 3245 (3NH), 1705, 1673 (2C=O) cm-1. 1H NMR (DMSO-d6) δ = 2.10 (s, 3H,CH3), 6.57(s, 1H, CH-pyrimidine) 7.26-8.19 (m, 10H, Ar-H+NH), 9.44 (s, 1H, NH), 10.01 (s, 1H, NH) and 11.89 (s, 1H, OH) ppm.13C NMR δ = 17.01, 60.91, 113.14, 119.42, 126.83, 127.28, 127.65, 128.83, 129.09, 129.44, 129.61, 130.49, 130.73, 158.11, 160.08, 185.47, 186.53. Anal. Calcd. For C19H17N3O3S (367.10) C, 62.11; H, 4.66; N, 11.44; S, 8.73. Found: C, 62.22; H, 4.76; N, 11.56; S, 8.82%.
4-(6-Methyl-2-thioxo-4-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carbox- amido) benzoic acid (3b, C20H19N3O3S).
Compound 3b was obtained as brown crystals from ethanol, Yield, (71%), m.p = 152 °C. IR (KBr) n = 3491 (OH), 3320, 3203, 3165 (3NH), 1701, 1639 (2C=O) cm-1 .1H NMR (DMSO-d6) δ = 2.20 (s, 3H, CH3), 2.28 (s, 3H, CH3), 5.94 (s, 1H, CH-pyrimidine), 6.58-7.24 (m, 10H, Ar-H+2NH), 9.24 (s, 1H, 1NH) and 11.99 (hump, 1H, OH) ppm.13C NMR δ = 18.93, 21.06, 56.52, 89.77, 104.56, 127.81, 130.18, 131.46, 138.36, 141.76, 143.40, 161.1, 171.45, 189.54. Anal. Calcd. For C20H19N3O3S (381.11): C, 62.97; H, 5.02; N, 11.02; S, 8.41. Found: C, 63.10; H, 5.10; N, 11.16; S, 8.53%.
4-(4-(4-Chlorophenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamido) benzoic acid (3c, C19H16ClN3O3S)
Compound 3c was obtained as brown crystals from ethanol, Yield (70%), m. p = 148-150 °C. IR (KBr) ν = 3423 (OH), 3364, 3292, 3167 (3NH), 1716, 1639 (2C=O) cm-1. 1H NMR (DMSO-d6) δ = 2.34 (s, 3H, CH3), 6.19 (s, 1H, CH), 6.60-7.91 (m, 10H, Ar-H+2NH), 10.00 (s,1H, NH) and 11.30 (hump, 1H, OH) ppm. Anal. Calcd. For C19H16ClN3O3S (401.06): C, 56.79; H, 4.01; Cl, 8.82; N, 10.46; S, 7.98. Found: C, 56.87; H, 4.13; Cl, 8.91; N, 10.59; S, 7.90%.
General procedure for preparation of compounds (4a,b)
A solution of 1 (0.01 mol), malononitrile or ethyl cyanoacetate (0.01 mol) in ethanol (30 mL), a few drops of piperdine was added and refluxed for 7 h. The solid product which produced on hot was collected by filtration and recrystallized from ethanol to give 4a, b.
4-(6-Amino-5-cyano-4-methyl-2-oxopyridin-1(2H)-yl) benzoic acid (4a, C14H11N3O3)
Compound 4a was obtained as brown crystals from ethanol yield, (70%), m. p = 250°C; IR(KBr) n = 3420 (OH, NH2), 2206 (C≡N), 1693, 1642 (2C=O) cm-1.1H NMR (DMSO-d6) δ = 2.19 (s, 3H, CH3), 5.68(s, 1H, CH-pyridine), 6.83 (s, 2H, NH2),7.37-8.09 (m, 4H, Ar-H) and 12.90 (hump, 1H, OH) ppm.13C NMR δ = 18.69, 69.79, 114.11, 120.00, 129.77, 130.23, 130.53, 140.01, 143.20, 160.91, 167.54, 182.15. Anal. Calcd. For C14H11N3O3 (269.26) C, 62.45; H, 4.12; N, 15.61. Found: C, 62.54; H, 4.23; N, 15.73 %.
4-(6-Amino-5-(ethoxycarbonyl)-4-methyl-2-oxopyridin-1(2H)-yl)benzoicacid(4b, C16H16N2O5)
Compound 4b was obtained as brown crystals from ethanol.Yield, (77%), m. p = 264°C; IR (KBr) δ = 3423 (OH); 3317, 3296 (NH2), 1684, 1633 (2C=O) cm-1.1H NMR (DMSO-d6) δ = 1.20 (t, 3H, CH3), 2.22 (s, 3H, CH3), 4.21 (q, 2H, CH2), 6.58-7.71 (m, 6H, Ar-H+NH2) and 12.59 (hump, 1H, OH) ppm.13C NMR δ =14.74, 16.16, 59.90, 78.00, 119.64, 130.64, 130.86, 131.65, 141.83, 147.88, 162.52, 165.80, 167.38, Anal. Calcd. For C16H16N2O5 (316.11) C, 60.75; H, 5.10; N, 8.86. Found: C, 60.84; H, 5.19; N, 8.93%.
4-((5-cyano-4-methyl-6-thioxo-1,6-dihydropyridin-2-yl) amino)benzoic acid (5, C14H11N3O2S)
A mixture of 1 (0.01 mol), cyanothiocetamide (0.01 mol) in ethanol (30 mL), a few drops of piperdinewere added and refluxed for 8h. The solid product which produced on hot was collected by filtration and recrystallized from ethanol to give 5 as brown crystals, yield (73%), m.p = 264°C, IR (KBr) n = 3498 (OH), 3214, 3189 (2NH), 2207 (C≡N), 1682(C=O) cm-1. 1H NMR (DMSO-d6) δ = 2.38 (s, 3H, CH3), 6.55 (s, 1H, CH-pyridinethione), 7.73-7.95(m, 5H, Ar-H +NH), 9.83 (s, 1H, NH) and 10.90 (hump, 1H, OH) ppm. 13C NMR δ = 15.50, 69.55, 119.51, 119.89, 120.43, 120.63, 130.62, 130.97, 131.65, 167.27, 182.36. Anal. Calcd. For C14H11N3O2S (285.06) C, 58.93, H; 3.89, N; 14.73; S, 11.24. Found: C, 58.02; H, 3.96; N, 14.85; S, 11.33%.
4-(5-Amino-4-cyano-3-methylthiophene-2-carboxamido) benzoic acid (6, C14H11N3O3S)
To a solution of 1 (0.01 mol), elemental sulfur, malononitrile and few drops of triethylamine 4 drops in absolute ethanol (30 mL) was refluxed for 8 h. The reaction mixture left to cool, the solid which formed collected by filtration, washed with ethanol, dried and recrystallized from ethanol to give 6 as brown crystals, yield (85%), m.p = 310°C. IR (KBr) δ = 3499(OH), 3379, 3327 (NH2), 3217 (NH), 2204 (C≡N), 1680,1639 (2C=O) cm 1.1H NMR (DMSO-d6) δ = 2.39 (s, 3H, CH3), 7.72-7.91 (m, 6H, Ar-H+NH2), 9.83 (s, 1H, NH) and 12.61 (s, 1H, OH) ppm.13C NMR δ = 15.41, 88.77, 113.17, 115.75, 119.91,125.76, 130.62, 141.82,143.53, 161.14, 165.92, 167.41. Anal. Calcd. For C14H11N3O3S (301.05): C, 55.80; H, 3.68; N, 13.95; S, 10.64%. Found: C, 55.91; H, 3.77; N, 14.13; S, 10.72%.
Preparation of compounds (7a-f): General procedure
A mixture of 1 (0.01 mol) with arylidine derivatives (0.01 mol) in ethanol (30 ml) was treated with few drops of piperdine and heated under reflux for 6 h. The solid product which produced on hot was collected by filtration and recrystallized from the proper solvent to give (7a-f).
4-(3-Acetyl-6-amino-5-cyano-2-oxo-4-phenylpyridin-1(2H)-yl) benzoic acid (7a, C21H15N3O4)
Compound 7a was obtained as yellow crystals from ethanol, Yield (85%), m.p = 132-134 °C; IR (KBr) n = 3500 (OH), 3395, 3334 (NH2), 2187 (C≡N), 1690,1634 (C=O) cm-1. 1H NMR (DMSO-d6) δ = 2.35 (s, 3H, CH3), 6.94-8.08 (m, 11H, Ar-H +NH2), and 10.65 (s, 1H, OH) ppm.13C NMR δ = 24.60, 56.50,113.31,114.38,119.22, 128.72, 129.11, 129.40, 130.77, 131.73, 151.15, 154.52, 159.66, 166.08, 167.18, 168.50. Anal. Calcd. For C21H15N3O4(373.11) C, 67.56; H, 4.05; N, 11.25%. Found: C, 67.65; H, 4.17; N, 11.30%.
4-(3-Acetyl-6-amino-4-(4-chlorophenyl)-5-cyano-2-oxopyridin-1(2H)-yl) benzoicacid (7b, C21H14ClN3O4)
Compound 7b was obtained as yellow crystals from ethanol, Yield (85%), m. p =144-146°C; IR (KBr) n = 3500 (OH), 3338, 3320 (NH2); 2204 (C≡N); 1698,1634 (C=O) cm-1. 1H NMR(DMSO-d6) δ = 2.25 (s, 1H, CH3), 6.91-8.06 (m,10H, Ar-H+NH2) and 12.80 (OH) ppm.13C NMR δ =14.42, 63.83, 117.93, 128.51, 128.83, 129.03, 129.11, 129.19, 129.40, 130.98, 132.78, 156.34, 165.66, 167.42, 203.85. Anal. Calcd. For C21H14ClN3O4(407.07): C, 61.85; H, 3.46; Cl, 8.69; N, 10.30%. Found: C, 61.94; H, 3.55; Cl, 8.69; N, 10.43%.
4-(3-Acetyl-6-amino-4-(4-chlorophenyl)-5-(ethoxycarbonyl)-2-oxopyridin-1(2H)-yl) benzoic acid (7c, C23H19ClN2O6)
Compound 7c was obtained as brown crystals from ethanol, yield (79%), m.p = 102-104°C; IR (KBr) n = 3516(OH), 3356, 3310 (NH2); 1713, 1700, 1692, 1636 (4C=O) cm-1. 1H NMR (DMSO-d6) δ = 1.21 (t, 3H, CH3), 1.25(s, 3H, CH3), 4.29(q, 2H, CH2), 7.33-8.07 (m, 10H, Ar-H +NH2) and 12.92 (hump, 1H, OH) ppm.13C NMR δ = 14.16, 14.41, 62.92, 86.21, 103.83, 113.07, 115.82, 119.30, 128.89, 129.18, 129.93, 130.72, 131.64, 132.87, 138.47, 154.12, 158.16, 162.09, 167.21. Anal. Calcd. For C23H19ClN2O6 (454.09) C, 60.73; H, 4.21; Cl, 7.79; N, 6.16. Found: C, 60.82; H, 4.33; Cl, 7.88; N, 6.29%.
4-(3-Acetyl-6-amino-5-cyano-4-(4-fluorophenyl)-2-oxopyridin-1(2H)-yl) benzoic (7d, C21H14FN3O4)
Compound 7d was obtained as yellow crystals from ethanol, Yield (85%), m.p = 180°C; IR (KBr) n = 3510 (OH), 3338, 3320 (NH2); 2206 (C≡N); 1698,1634 (C=O) cm-1. 1H NMR (DMSO-d6) δ = 2.27 (s, 1H, CH3), 6.56-7.64 (m, 10H, Ar-H +NH2) and 12.54 (s, 1H, OH) ppm.13C NMR δ = 22.60, 113.06, 116.04, 119.28, 129.35, 130.44, 130.61, 130.72, 130.97, 131.15, 131.24, 131.24, 131.42, 131.65, 153.57, 153.87, 167.22, 182.60. Anal. Calcd. For C21H14FN3O4 (391.10) C, 64.45; H, 3.61; F, 4.85; N, 10.74. Found: C, 64.54; H, 3.70; F, 4.96; N, 10.84%.
4-(3-Acetyl-6-amino-5-cyano-2-oxo-4-(p-tolyl) pyridin-1(2H)-yl)benzoic acid (7e, C22H17N3O4)
Compound 7e was obtained as yellow crystals from ethanol, Yield (85%), m.p = 158-160°C; IR (KBr) n = 3510 (OH), 3338, 3320 (NH2); 3250 (NH), 2206 (C≡N); 1698, 1634 (3C=O) cm-1. 1H NMR (DMSO-d6) δ = 2.27, 2.33(2s, 6H, 2CH3), 6.66 (s, 2H, NH2), 7.26-8.17 (m, 8H, Ar-H) and 12.73 (s, 1H, OH) ppm. 13C NMR δ = 18.94, 21.13, 113.09, 119.31, 128.70, 129.22, 130.80, 131.65, 133.96, 139.90, 142.00, 167.21, 174.37,174.96, 177.17. Anal. Calcd. For C22H17N3O4 (387.12): C, 68.21; H, 4.42; N, 10.85 %. Found: C, 68.29; H, 4.51; N, 10.93%.
4-(3-Acetyl-6-amino-5-cyano-4-(4-hydroxyphenyl)-2-oxopyridin-1(2H)-yl) benzoic acid (7f, C21H15N3O5)
Compound 7f was obtained as yellow crystals from ethanol, Yield (85%), m.p = 160 °C; IR (KBr) n = 3486 (OH), 3327, 3305 (NH2); 2204 (C≡N); 1712, 1693, 1634 (3C=O) cm-1. 1H NMR (DMSO-d6) δ = 2.19 (s, 3H, CH3), 5.62 (s, 2H, NH2), 6.65-8.09 (m, 10H, Ar-H +NH2) and 11.85 (s, 1H, OH) ppm. 13C NMR δ = 18.29, 62.42, 115.68, 116.29, 128.05, 128.40, 129.15, 129.92, 130.00, 131.57, 132.21, 135.19, 158.93, 166.01, 167.35, 170.47, 172.10. Anal. Calcd. For C21H15N3O5 (389.10) C, 64.78; H, 3.88; N, 10.79. Found: C, 64.86; H, 3.97; N, 10.87%.
4-(3-Acetyl-2-oxoquinolin-1(2H)-yl) benzoic acid (9, C18H13NO4)
A mixture of 1 (0.01 mol) with salicylaldehyde (0.01 mol), Pyridine (10 ml) heated under reflux for 13 h. The solid product which produced on hot was collected by filtration and recrystallized from the proper solvent to give 9 as brown crystals. Yield (65%), m.p = 164-166 °C; IR (KBr) n = 3482 (OH), 1709, 1680, 1635 (3C=O) cm-1.1H NMR (DMSO-d6) δ = 2.59 (s, 3H, CH3), 6.58-7.38 (m, 9H,Ar-H) and 12.50 (hump, 1H, OH) ppm.13C NMR δ = 18.95, 113.44, 116.57, 125.41, 126.83, 130.68, 131.21, 131.65, 131.84, 134.94, 144.00, 144.51, 147.46, 153.03, 154.68, 167.92, 172.05, 187.31. Anal. Calcd. For C18H13NO4 (307.30) C, 70.35; H, 4.26; N, 4.56. Found: C, 70.46; H, 4.37; N, 4.63%.
4-(3-Acetyl-6-(4-bromophenyl)-2-oxo-4-(p-tolyl) pyridin-1(2H)-yl)benzoic acid (10, C27H20BrNO4)
To a solution of 1 (0.01 mol) in ethanol (40 mL) containing catalytic amount of piperidine 4 drops, 1-(4-bromophenyl)-3-(p-tolyl)prop-2-en-1-one (0.01 mol) was added. The reaction mixture was heated under reflux for 5 h. The solid product formed on heating was collected by filtration, recrystallized from the proper solvent to give 10 as orange crystals. yield (83%),; m.p = 140-142 °C. IR (KBr) n = 3490 (OH), 1703,1654, 1636 (3C=O) cm-1.1H NMR (DMSO-d6) δ = 2.21 (s, 3H, CH3), 2.36 (s, 3H, CH3), 7.12-7.91 (m, 13H, Ar-H) and 12.76 (s, 1H, OH) ppm.13C NMR δ = 21.56, 121.21, 127.61, 129.45, 130.01, 130.94, 132.26, 132.35, 137.16, 141.33, 145.05, 167.26, 175.21, 188.76. Anal. Calcd. for C27H20BrNO4 (501.06) C, 64.55; H, 4.01; Br, 15.91; N, 2.79. Found: C, 64.64; H, 4.13; Br, 15.98; N, 2.87%.
4-(4-Hydroxy-2-phenyl-6-thioxo-1,6-dihydropyridine-3-carboxamido)-benzoic acid (11, C19H14N2O4S)
To a solution of 1 (0.01 mol), in dry acetone (30 ml) and Phenyl isothiocyanate (0.01 mol), was refluxed for 8 h. The solvent is left to evaporation, the solid which formed collected to give 11 as yellow crystals. yield (85%), m.p = 206-208 °C. IR. (KBr) n = 3499(OH), 217(NH), 1680,1639 (2C=O) cm-1.1H NMR (DMSO-d6) δ = 7.19-8.01 (m,12H, Ar-H+CH +2NH) and 11.56, 12.77 (s,2H, OH) ppm.13C NMR δ =118.88, 123.92, 128.61, 128.92, 129.16, 130.39, 132.57, 133.56, 142.41, 167.14, 168.67, 179.51. Anal. Calcd. For C19H14N2O4S (366.07): C, 62.28; H, 3.85; N, 7.65; S, 8.75. Found: C, 62.37; H, 3.91; N, 7.71; S, 8.84%.
4-((5-Methyl-2-oxo-3-(p-tolyldiazenyl)-1,2-dihydropyrazolo[1,5-a] pyrimidin-7-yl) amino) benzoic acid (13, C21H18N6O3)
In a round-bottomed flask attached to a Dean and Stark constant water separator which is connected to a reflux condenser are placed a mixture of 1 (0.05 mol), 5-amino-4-(p-tolyldiazenyl)-1H-pyrazol-3(2H)-one (0.05 mol), 100 ml of benzene, and 1 ml of glacial acetic acid. The flask is heated in an oil bath at about 125 °C, and the water which distils out of the mixture is removed at intervals. Refluxing is continued until no more water separates and then for an additional 30 minutes. The benzene is then distilled under reduced pressure, The solid product formed was filtered off and recrystallized from the proper solvent to give 13 as red crystals. Yield (63%), m.p = 230 °C. IR. (KBr) n= 3500 (OH) 3250, 3176 (2NH), 1670, 1632 (2C=O) cm 1. 1H NMR (DMSO-d6) δ = 1.23, (s, 1H, CH3), 2.29 (s, 1H, CH3), 6.54-7.93 (m, 10H, Ar-H+NH+CH), 10.56 (s, 1H, NH) and 12.96 (s, 1H, OH) ppm. 13C NMR δ = 20.89, 21.17, 115.60, 117.49, 119.18, 130.28, 130.79, 131.66, 134.04, 137.58, 140.00, 150.34, 153.57, 154.45, 156.14, 160.55,167.39, 167.94. Anal. Calcd. For C21H18N6O3 (402.14): C, 62.68; H, 4.51; N, 20.88 %. Found: C, 62.78; H, 4.61; N, 20.68 %.
References
- Krátký M, Konečná K, Janoušek J, Michaela B, Janďourek O, et al. (2020) 4-Aminobenzoic Acid Derivatives Converting Folate Precursor to Antimicrobial and Cytotoxic Agents. Biomolecules 10(1): 9-28.
- Saavedra JE, Srinivasan A, Buzard GS, Davies KM, Waterhouse KI, et al. (2006) PABA/NO as an Anticancer Lead: Analogue Synthesis, Structure Revision, Solution Chemistry Reactivity Toward Glutathione and in Vitro Activity. J Med Chem 49(3): 1157-64.
- Correa-Basurto J, Alcántara IV, Espinoza-Fonseca LM, Trujillo-Ferrara JG (2005) p–Aminobenzoic acid derivatives as acetylcholinesterase inhibitors. Eur J Med Chem 40(7): 732-735.
- Little GR; US Patent, (2002), 6,436,660.
- Bruze M, Gruvberger B, Thulin I (1990) PABA Benzocaine and Other PABA Esters in Sunscreens and After-Sun Products. Photodermatol Photoimmunol Photomed 7(3): 106-108.
- Phoon CW, Yong P, Ting AE, Yeob SL, Sima MM (2001) Biological evaluation of hepatitis c virus helicase inhibitors. Bioorg Med Chem Lett 11(13): 1647-1650.
- Boularot A, Giglione C, Petit S, Duroc Y, De Sousa RA, et al. (2007) discovery and Refinement of a New Structural Class of Potent Peptide Deformylase Inhibitors. J Med Chem 50(1): 10-20.
- Pichota A, Duraiswamy J, Yin Z, Keller TH, Alam J, et al. (2008) Peptide Deformylase Inhibitors of Mycobacterium Tuberculosis synthesis Structural Investigations and Biological Results. Bioorg Med Chem Lett 18(24): 6568-6572.
- Abdel-Hafez E, Abuo-Rahma GEAA, Abdel-Aziz M, Radwan MF, Farag HH (2009) design, synthesis and biological investigation of certain pyrazole-3-carboxylic acid derivatives as novel carriers for nitric oxide. Bioorg Med Chem 17(11): 3829-3837.
- Phuong T, Khac-Minh T, Van Ha NT (2004) Synthesis and antifungal activities of phenylenedithioureas. Bioorg Med Chem Lett 14(3): 653-656.
- Hussein AM, Khames AA, El-Adasy AA, Atalla AA, Abdel-Rady M, et al. (2020) design, synthesis and biological evaluation of new 2-aminothiazole scaffolds as phosphodiesterase type 5 regulators and COX-1/COX-2 inhibitors. RSC Adv 10: 29723-29736.
- Hussein AM, El-Adasy AA, Khames AA, Atalla AA, Abdel-Rady M (2017) 3-Oxobutanamides in Heterocyclic Synthesis, Synthesis Approaches for new Pyridines, Pyrimidines and their Fused Derivatives. Chemistry Select 2(4): 1625-1629.
- Hossbach R, Lettau H, Nuhn P, Schneider R, Stenger P, Stiebitz B (1991) Pharmazie, , 46, 412–415.
- Ibrahim DA, El-Metwally AM (2010) Design synthesis and biological evaluation of novel pyrimidine derivatives as CDK2 inhibitors. Eur J Med Chem 45(3): 1158-1166.
- Gein VL, Kholkin IV, Zamaraeva TM, Voronina EV, Vakhrin MI (2012) synthesis and antimicrobial activity of N,6-diaryl-4-methyl-2-thioxo-1,2,3,6-tetrahydropyrimidine-5-carboxamides. Pharmaceutical Chemistry Journal 46(2), 114-117.
- Makarov VA, Ryabova OB, Alekseeva LM, Shashkov AS, Granik VG (2003) synthesis of derivatives of pyrazolo[3,4-d] pyrimidines by reaction of 3,5-bis(dimethylaminomethylene)amino-4-methoxycarbonylpyrazole and 4-cyanopyrazole with amines. Chemistry of Heterocyclic Compounds 39(2): 238–243.
- Hussein AM, Gad-Elkareem MAM, El-Adasy AAM, Khames AA, Othman IMM (2012) β-Oxoanilides in Heterocyclic Synthesis: Synthesis and Antimicrobial Activity of Pyridines, Pyrans, Pyrimidines and Azolo, Azinopyrimidines Incorporating Antipyrine Moiety. International Journal of Organic Chemistry 2(4): 341-351.
- Ahmed SA, Hussein AM, Hozayen WG, El-Ghandour AH, AbdEl-Hamid AO (2007) Synthesis of some Pyrazolopyrimidines as Purine Analogues. J Heterocycl Chem 44(4): 803-810.
- Parekh J, Nair R, Chanda S (2005) Preliminary screening of some folklore medicinal plants from western India for potential antimicrobial activity. Indian Journal of Pharmacology 37(6): 408-409.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...