Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Research Article(ISSN: 2637-4706) 
Successively Substituting an Additional 4-(2-Aminoethyl) Aniline Group in Fabricated Isoindoline-1,3-Dione Scaffold Enhances the Antimicrobial Potency: Part II of Research Volume 2 - Issue 2
Debarshi Kar Mahapatra1*, Mohamad Taleuzzaman2, Santosh S Chhajed3
- 1Department of Pharmaceutical Chemistry, Dadasaheb Balpande College of Pharmacy, India
- 2Department of Pharmaceutical Chemistry, Glocal School of Pharmacy, Glocal University, India
- 3Department of Pharmaceutical Chemistry, MET’s Institute of Pharmacy, India
Received: August 16, 2018; Published: August 27, 2018
Corresponding author: Debarshi Kar Mahapatra, Department of Pharmaceutical Chemistry, Dadasaheb Balpande College of Pharmacy, Nagpur 440037, Maharashtra, India
DOI: 10.32474/DDIPIJ.2018.02.000132
Abstract
The irrational use of antimicrobial agents for several decades has led to the drug-resistance among the patient population. Overcoming the present drug-resistance is a major challenge for modern day scientists. In order to counter this problem, several approaches have been utilized, one of which involve drug design and discovery, where new classes or unexplored chemical moieties are explored for a particular activity, either serendipitously or quite rationally. Learning lessons from the previously synthesized four compounds (Part-I of Research), we have now (Part-II of Research) designed two compounds (3 and 5) by incorporating an additional 4-(2-aminoethyl)aniline group in the fabricated isoindoline-1,3-dione scaffold by successive synthetic step utilizing similar protocol and screened them against the above four mentioned pathogenic microbes in a similar way. The present exploration is an attempt to rationally overcome the microbial drug resistance by the efficacious and potent nature of the compounds. Compound (5) exhibited the highest activity against A. niger with ZOI diameter of >29mm. All the compounds showed more or less nearly the same activity. From this study, it may be concluded that substituting the amine/amide-based components leads to an increase in the potency of the compared along with better-anti-fungal activity. Therefore, the current exploration helped to design the compounds which have better potency than our previous reports and also providing imperative knowledge regarding the selection of the substituents. The encouraging results will further motivate us in developing novel and better inhibitors of phthalimide scaffold in the future.
Keywords: Antimicrobial; Antifungal; Antibacterial; Phthalimide; Isoindoline; Synthesis
Introduction
The irrational use of antimicrobial agents for several decades has led to the drug-resistance among the patient population [1]. Overcoming the present drug-resistance is a major challenge for modern day scientists. In order to counter this problem, several approaches have been utilized, one of which involve drug design and discovery, where new classes or unexplored chemical moieties are explored for a particular activity, either serendipitously or quite rationally [2]. In the same way, in our previous research (Part-I of Research), based on the research evidence that phthalimide based compounds have gained adequate attention as anti-infective (anti- fungal, anti-microbial, anti-retroviral, anti-influenza, anti-malarial, and anti-tubercular) very recently [3]. Previously, we developed some potential phthalimide based compounds (2-((2-(4-substituted- phenyl) hydrazinyl) methyl) isoindoline-1,3-dione) derived from N-chloromethyl phthalimide by reacting with aromatic hydrazine derivatives and screened for anti-microbial studies against two prominent species of bacteria; Escherichia coli and Staphylococcus aureus, and the fungal species, Candida albicans and Aspergillus niger [4]. The observed results were not very impressive, in terms of potency (50 μg/mL) as compared with the standard drugs. All the compounds demonstrated both bactericidal and fungicidal activity in a moderate depth. However, the measured activities were observed to be nearly the same and a very reasonable conclusion was not able to draw from the previous study at all. Therefore, learning lessons from the previously synthesized four compounds, we have now (Part-II of Research) designed two compounds by incorporating an additional 4-(2-aminoethyl)aniline group in the fabricated isoindoline-1,3-dione scaffold by successive synthetic step utilizing similar protocol and screened them against the above four mentioned pathogenic microbes in a similar way. The present exploration is an attempt to rationally overcome the microbial drug resistance by the efficacious and potent nature of the compounds.
Materials and Methods
Chemicals and Instrumentation
The starting material, 2-((2-(4-chlorophenyl)hydrazinyl) methyl)isoindoline-1,3-dione (1) is a previously reported component by our research team. The reactants; 4-(2-aminoethyl) aniline (2) and 4-(2-(methylamino)ethyl)aniline (4) were purchased from Sigma Aldrich, Germany through a local vendor. The final compounds and the intermediates were analyzed using spectroscopic methods: Fourier-Transformed Infrared (FTIR) study, employing on Shimadzu® IRAffinity-1 instrument; 1H (proton)-NMR study was performed on Bruker Avance-II instrument, making use of an internal standard, tetramethylsilane; mass studies were executed on MICROMASS Q-TOF instrument. Following the spectroscopic studies, the elemental analysis was performed on PerkinElmer Elemental Analyzer 2400. The advancement of the chemical reaction was examined by Merck precoated Silica gel-G TLC plates.
Synthesis of Target Compounds
The synthesis involved chemical reaction of 2-((2-(4-chlorophenyl)hydrazinyl) methyl) isoindoline-1,3-dione (1) with two different reactants; 4-(2-aminoethyl)aniline (2) and 4-(2-(methylamino)ethyl)aniline (4) to produce destination compounds (3 and 5). The reaction took place at a low temperature in which a hydrochloride moiety (HCl) gets released to the reaction mixture to form the final molecules. The Cl moiety was released from (1) and the corresponding hydrogen atom was liberated from the reactants (2 and 4). Scheme 1 illustrates the present chemical synthesis protocol.
Scheme 1: Outline for the synthesis of 2-((2-(4-((4-(2-(substituted) phenyl) amino) phenyl) hydrazinyl) methyl) isoindoline- 1,3-diones.
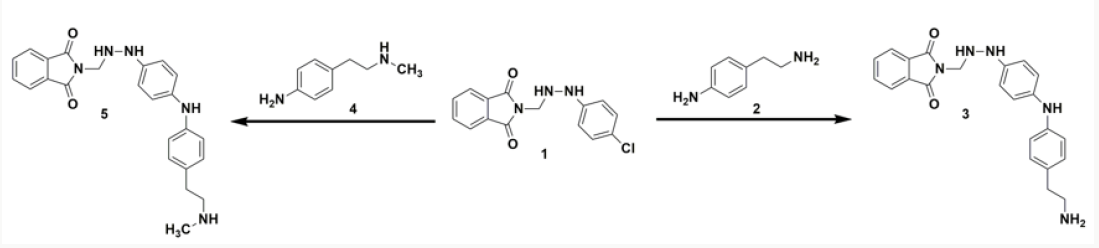
Synthetic protocol for 2-((2-(4-((4-(2-(substituted) phenyl) amino) phenyl) hydrazinyl) methyl) isoindoline- 1,3-diones
Based on our previous synthetic protocol, ethanol-methanol solution of 0.01 M of 2-((2-(4-chlorophenyl)hydrazinyl) methyl) isoindoline-1,3-dione (1) was made to react with equimolar concentrations of 4-(2-aminoethyl)aniline (2) and 4-(2-(methylamino)ethyl)aniline (4) by adding drop wise at a lowered temperature between 0-5°C. The reaction content was stirred employing magnetic stirrer for 5 hrs at room temperature. The desired solid products (3 and 5) were separated out, filtered under vacuum utilizing Buchner funnel, and washed carefully with 10% aqueous sodium bicarbonate (NaHCO3). The products were dried under vacuum and further recrystallized with methanol acquire the pure crystalline content.
2-((2-(4-((4-(2-aminoethyl) phenyl) amino) phenyl) hydrazinyl) methyl) isoindoline-1,3-dione (3)
67% yield; FTIR (KBr) υ (cm-1): 3384 (-NH2), 3191 (-NH, stretching), 3077 (C-H, aromatic), 1683 (C=O), 1614 (C=C, aromatic), 1553 (-NH, bending), 1328 (C-N); 1H NMR (δ, ppm, CDCl3): 6.7-8.2 (Aromatic, 12H), 5.22 (Amine, 2H), 4.58 (8, 2H), 4.19 (10, Amide), 3.08 (20, 2H), 2.84 (21, 2H). MS: M+ 401. Anal. Calcd. for C23H23N5O2: C, 68.81; H, 5.77; N, 7.97. Found: C, 68.24; H, 5.21; N, 7.32.
2-((2-(4-((4-(2-(methylamino)ethyl)phenyl)amino)phenyl) hydrazinyl)methyl)isoindoline-1,3-dione (5)
74% yield; FTIR (KBr) υ (cm-1): 3172 (-NH, stretching), 3104 (C-H, aromatic), 1694 (C=O), 1619 (C=C, aromatic), 1578 (-NH, bending), 1307 (C-N); 1H NMR (δ, ppm, CDCl3): 6.4-8.3 (Aromatic, 12H), 4.66 (8, 2H), 4.23 (10, Amide), 3.41 (23, 3H), 3.13 (20, 2H), 2.89 (21, 2H). MS: M+ 415. Anal. Calcd. for C24H25N5O2: C, 69.38; H, 6.06; N, 16.86. Found: C, 68.99; H, 5.71; N, 16.37.
Antimicrobial screening
2-((2-(4-((4-(2-aminoethyl) phenyl) amino) phenyl) hydrazinyl) methyl) isoindoline-1,3-dione derivatives were screened for anti-microbial studies against Escherichia coli (E. coli, MTCC 2961), Staphylococcus aureus (S. aureus, MTCC 3160), Candida albicans (C. albicans, MTCC 227), and Aspergillus niger (A. niger, MTCC 277). Disc diffusion method was the protocol for estimating anti-microbial activity. The microbial species were initially in nutrient broth media at 37°C for 24 hrs, followed by culturing the cells in specific agar plates utilizing the Muller Hinton agar medium (anti-bacterial study) and potato dextrose agar medium (anti-fungal study) under a laminar flow cabinet. The experimental compounds were dissolved in DMSO, soaked using a Whatman filter paper, and cultured employing media containing plates. Incubation was performed at 37ºC for 24 hrs for anti-bacterial study and 28±2ºC for 72 hrs for anti-fungal study. Ciprofloxacin was made use as the standard compound for anti-bacterial study and similarly, fluconazole was utilized for the anti-fungal study. The activity of the produced compounds was found out and expressed as the zone of inhibition diameter (in mm). DMSO was used as a negative control [5]. The agar streak dilution method was employed for estimating minimum inhibitory concentration (MIC) where the microbial suspension of 105 CFU/mL concentration was applied to the petri dish using DMSO for serial dilution. The test compounds were suspended on the microbial suspension into the petri dish, up to 4-5 mm depth, at a temperature of 40-50ºC. The microbial plates were incubated at 37±1ºC. The MIC values were determined in triplicate manner and expressed as the average value [6].
Results and Discussion
Chemistry
The obtained spectra revealed several imperative facts which helped confirmation of the final structures of the synthesized compounds. The first and sure shot confirmation of the formation of the molecule (3) was the presence of amine which was noticed at >3300 cm-1 in FT-IR spectra. Apart from it, several imperative features of phthalimide scaffold were seen in FT-IR spectra. The components C=O, C=C, C-N, and C-H of phthalimide scaffold were located chiefly. The amide (-NH) portion was positioned prominently adjacent to the amine at >3150 cm-1 in FT-IR spectra. The compound (5) had no specific amine group and therefore the FT-IR spectra did not show any characteristic peak in the range of 3200-3350 cm-1 in FTIR spectra. When compared with the FT-IR spectra of the starting material (1), distinct differences were observed. The presence of no amine group was a discrete feature which helped to distinguish from compound (3) and was an appropriate evidence for the creation of the proposed compounds. The 1H-NMR of the molecules additionally supported the above-mentioned data completely. The amide components were predominantly found at 4 ppm. The phthalimide scaffold was substantiated by the –CH2- fragment present at 5 ppm. The presence of the three prominent aromatic rings was essentially located in the range of 6.4-8.3 ppm. The main difference between both the compounds was clearly examined from the spectra where the amine protons of compound (3) were noticed at 5.22 ppm and protons of the methyl group of compounds (5) were perceived at 3.41 ppm. Furthermore, the presence of alkyl fragments in the final structures at 2.8-3.1 ppm represented that the reactant reacted with the starting material successfully. The mass spectra displayed few crucial aspects which helped in the characterization. The fabricated compounds exhibited base peaks in the mass spectra which exactly corresponded with the molecular weight of the compounds. The close agreement between theoretical values and observed values of carbon, hydrogen, and nitrogen ratios supported the formation of the two designated compounds.
Antimicrobial study
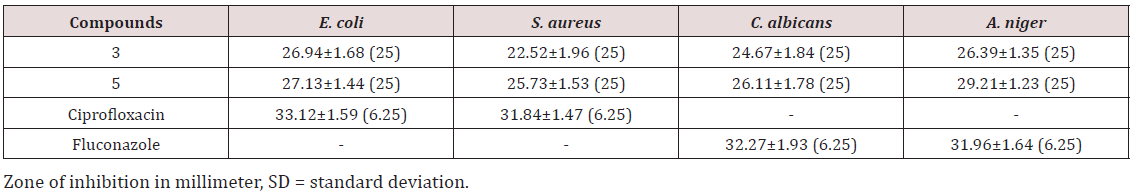
An impressive anti-microbial activity was demonstrated by the two compounds against the four species of microbes. Both the compounds displayed excellent activity against E. coli and A. niger with ZOI diameter of >25 mm. Compound (5) exhibited the highest activity against A. niger with ZOI diameter of >29 mm. All the compounds showed more or less nearly the same activity. Both the molecules expressed average or moderate activity against S. aureus. A noteworthy anti C. albicans was seen in the anti-microbial screening (Table 1). Both the molecules did not show activity better or comparable activity than the standard compound. The MIC values of both the compounds against the pathogen species were found to be 25 μg/mL, which indicated that the synthesized compounds are potent enough and have good biological activity. As compared with our previous research, it was vigilantly noticed that substituting the amine/amide based components leads to increasing in the potency of the compared along with better-anti-fungal activity. Therefore, the current exploration helped to design the compounds which have better potency than our previous reports, and also providing imperative knowledge regarding the selection of the substituents.
Conclusion
The present research represented a continuous effort, therefore, presented as Research Part-II where a search for better potent molecules is aimed to design the most effective inhibitor of phthalimide scaffold. At present, drug resistance offers a great challenge for clinicians for long-duration, safe, and effective treatment. While walking towards the pathway of overcoming the problems, it was observed that both the compounds displayed excellent activity against E. coli and A. niger with ZOI diameter of >25 mm. Compound (5) exhibited the highest activity against A. niger with ZOI diameter of >29 mm. All the compounds showed more or less nearly the same activity. Both the molecules expressed average or moderate activity against S. aureus. From this study, it may be concluded that substituting the amine/amide-based components leads to increasing in the potency of the compared along with better-anti-fungal activity. Therefore, the current exploration helped to design the compounds which have better potency than our previous reports and also providing imperative knowledge regarding the selection of the substituents. The encouraging results will further motivate us in developing novel and better inhibitors of phthalimide scaffold in the future.
References
- Cohen FL, Tartasky D (1997) Microbial resistance to drug therapy: a review. Am J Infec Contr 25(1): 51-64.
- Mahapatra DK, Bharti SK (2017) Handbook of Research on Medicinal Chemistry, New Jersey: Apple Academic Press.
- Sharma U, Kumar P, Kumar N, Singh B (2010) Recent advances in the chemistry of phthalimide analogues and their therapeutic potential. Mini Rev Med Chem 10(8): 678-704.
- Mahapatra DK, Taleuzzaman M, Gupta SD (2018) Novel 2-((2-(4-substituted-phenyl) hydrazinyl) methyl) isoindoline-1,3-dione derivatives as promising anti-microbial agents: Part-I of Research. Pharmacol Pharm Rep (Communicated).
- Mahapatra DK, Shivhare RS, Joseph TM (2017) Design and characterization of Murrayanine linked Isoxazole derivatives: Novel class of bacteriocidal agents. Int J Res Drugs Pharm Sci 1(1): 11-15.
- Telrandhe R, Mahapatra DK, Kamble MA (2017) Bombax ceiba thorn extract mediated synthesis of silver nanoparticles: Evaluation of anti – Staphylococcus aureus activity. Int J Pharm Drug Anal 5(9): 376-379.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...