
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Short Communication(ISSN: 2637-4706) 
»N2B-Patch« – Nose-To-Brain Delivery of an Active Pharmaceutical Ingredient via the Olfactory Region Volume 1 - Issue 4
Carmen Gruber Traub* and Jenny Ullrich
- Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB, Stuttgart, Germany
Received: May 04, 2018; Published: May 10, 2018
Corresponding author: Carmen Gruber Traub, Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB, Nobelstrasse 12, 70569 Stuttgart, Germany
DOI: 10.32474/DDIPIJ.2018.01.000116
Abstract
Within the EU funded project »N2B-patch« eleven partners from eight countries aim to develop an innovative technology for the nose-to-brain delivery of an active pharmaceutical ingredient via the olfactory region for the regenerative treatment of multiple sclerosis using novel multi-functional biomaterials combined with a medical device. The four year EU-funded research involves an innovative method of bypassing the blood-brain barrier by delivering a potential multiple sclerosis drug with a special medical device via the nose directly to the brain.
Keywords: nose-to-brain, multiple sclerosis, multi-functional biomaterial, drug delivery, API-loaded particles
Short Communication
Within the EU funded project »N2B-patch« eleven partners from eight countries aim to develop an innovative technology for the nose-to-brain delivery of an active pharmaceutical ingredient via the olfactory region for the regenerative treatment of multiple sclerosis using novel multi-functional biomaterials combined with a medical device. The four year EU-funded research involves an innovative method of bypassing the blood-brain barrier by delivering a potential multiple sclerosis drug with a special medical device via the nose directly to the brain. The World Health Organisation (WHO) estimates that more than one billion people worldwide are suffering from neurological disorders [1]. The therapy of central nervous system (CNS) diseases like multiple sclerosis (MS) is still an unmet medical need due to the low CNS bioavailability of innovative drugs like antibodies. Hence, a safe and efficient drug delivery platform technology for CNS active molecules is crucial and therefore needed.
MS is one of the most common long-term conditions affecting the CNS and the main cause of non-traumatic disability in young adults. MS is a neurodegenerative disease, a group of conditions that include Parkinson’s and Alzheimer’s. It affects more than 700,000 people in Europe. MS is an inflammatory, demyelinating disease and the leading cause of non-traumatic neurological disability in young adults and it is estimated to affect 2.3 million people worldwide; prevalence is particularly high in Europe and North America, with rates over 100 cases per 100,000 [2]. Current standard therapies of MS include anti-inflammatory and symptomatic treatments. Although, relapse occurrence and duration are reduced by means of these therapies, no regeneration is achieved and there are no treatments for the progressive forms of MS. Therefore, MS is still a degenerative disease that leads to disability of patients in their most productive years. A key factor in the development of functional ingredients or active substances is their transport to their target location. Pharmaceutical active ingredients (APIs) are normally distributed via the blood, either directly by injection into the bloodstream or indirectly, for example through the digestive tract after oral administration. However, in many diseases, for example of the CNS, it is of decisive importance to transport the API as efficiently as possible to the required target site. An example of this is the treatment of MS, where the pharmaceutical agents have to produce their effect above all in the CNS. This is especially difficult to achieve in the usual way via the blood due to special protective mechanisms such as the blood-brain barrier (BBB). The direct transport route from the nasal cavity to the brain, bypassing the BBB, could potentially offer an exciting method for CNS drug delivery. The nose-to-brain (N2B) transport of therapeutic drugs is a scientifically well-known and clinically proven alternative route of administration as the so-administered drugs can bypass the BBB.
The therapeutic system within »N2B-patch«will consist of the active agent itself, a formulation containing the API, a hydrogel as carrier material for the formulation, and a suitable applicator (Figure 1). As the project coordinator, the Fraunhofer IGB is involved in the development of a medical therapy for the administration of active ingredients via the olfactory region in »N2B-patch«. In the project the Fraunhofer IGB is concentrating on the formulation of the particles containing the active substance and the embedding of these particles into hydrogels. Various particle technologies are used to formulate micro- and nanoparticles loaded with APIs (Figure 2). Bio based or biodegradable polymers are chosen as the base material for the particles. Spray drying is a suitable process to formulate different classes of molecules like small organic compounds or biomolecules and spray drying is one method that can be used for an effective encapsulation of biomolecules like insulin or interferon without losing the bioactivity of the encapsulated protein [3]. Two- and three-fluid nozzles are used for the particle preparation. For the one-step preparation of core-shell particles by spray drying the three-fluid nozzle has already been successfully integrated into the process.
Figure 1: An ensemble composed of biomaterial-based drug particles and an innovative active pharmaceutical ingredient (API) for multiple sclerosis (MS) treatment (left), the hydrogel formulation as a carrier (middle), the applicator device as integral part of the functional and minimally-invasive system (right).
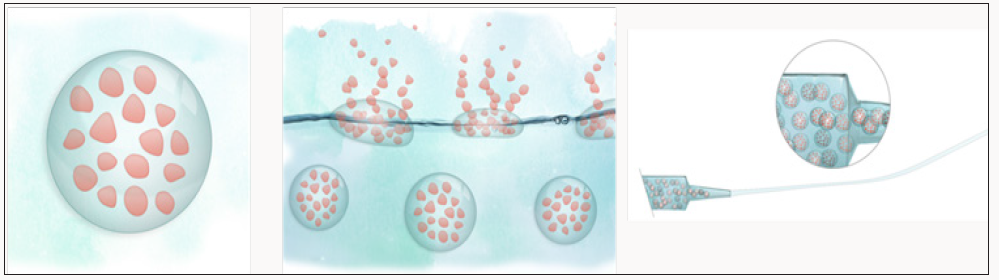
Figure 2: Scanning electron microscopic (SEM) images of different particles: Protein-loaded Insulin particles (left), proteinloaded chitosan particles (right), both prepared by a spray drying process and PEG-PLGA particles prepared by emulsion technologies (middle).
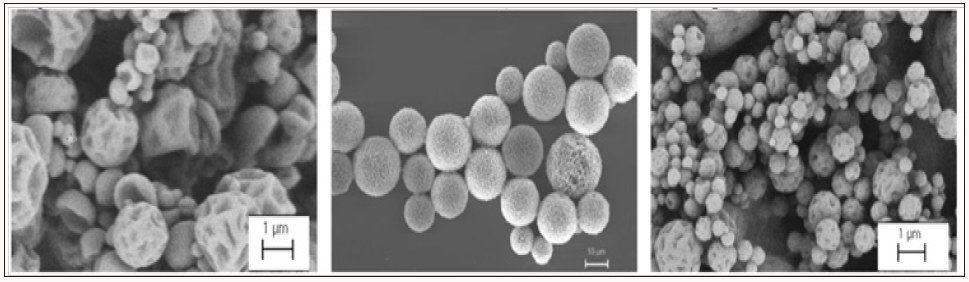
Various biobased polymers like chitosan, gelatine, alginate and different cross-linkers (covalent and non-covalent) in the production of particles are currently being tested. The biopolymer chitosan, known to interact with the mucosal surface, will be used as a Mucoadhesive particle material or as Mucoadhesive coating. Chitosan can, like other cationic molecules, interact with the mucus surface. The release properties of the APIs and particles can further be optimized by varying the particle material, the cross linking degree or by the additional coating of the particles. The developed API-loaded particles can then be integrated into a hydrogel matrix and administered directly to the olfactory cleft with the new intranasal application system. The technology developed by the »N2B-patch« project may also be suitable for the use of different pharmaceutical ingredients. The envisaged direct drug delivery platform may also support the treatment of other demyelinating disorders and other CNS indications, e.g. stroke, neurodegenerative diseases or tumours.
Acknowledgment
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 721098 - »N2B-patch«. The project started on 1st January 2017 with duration of 48 months. More information can be found on the project websitewww.n2b-patch.eu.
References
- (2007) Neurological Disorders: Public health challenges, World Health Organization.
- (2008) Atlas multiple sclerosis resources in the world 2008. WHO Library Cataloguing-in-Publication Data.
- Gruber Traub C, Burger Kentischer A, Gretzinger S, Hirth T, Weber A (2012) Chemie Ingenieur Technik 84: 343-348.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...














