Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Research Article(ISSN: 2637-4706) 
Effect of Superdisintegrants on the Enhancement Dissolution Characteristics for Lamotrigine Volume 3 - Issue 4
Raghavendra Kumar Gunda1* and Prasad Rao Manchineni2
- 1Department of Pharmaceutics, M.A.M college of Pharmacy, India
- 2Department of Pharmaceutical Analysis, M.A.M college of Pharmacy, India
Received: March 16, 2020; Published: April 20, 2020
Corresponding author: Raghavendra Kumar Gunda, Department of Pharmaceutics, M.A.M college of Pharmacy, India
DOI: 10.32474/DDIPIJ.2020.03.000166
Abstract
The purpose of present investigation to study the effect of various superdisintegrants on the dissolution characteristics of Lamotrigine by formulating oral disintegrating tablets (ODT). Lamotrigine, an antiepileptic agent, belongs to type –II as per Biopharmaceutical Classification System (BCS) exerts Low aqueous solubility and high permeability behavior. ODT formulations of Lamotrigine were prepared using different quantities of Sodium Starch Glycolate (SSG) & Crospovidone (CP) employed as Superdisintegrants by Direct Compression technique as per 32 Factorial Design. Nine trials were formulated by considering amount of Crospovidone (X1), amount of SSG (X2) as Independent variables. The developed Formulations were evaluated for Pharmaceutical Product Performance. The t10%, t50%, t90%, Wetting time, Disintegration time were taken as Dependant variables. Results shows that all the formulations were lie within the acceptance criterion and the In-vitro dissolution profiles were subjected to kinetic modeling. The Polynomial equations were derived for dependant variables and are verified for validity by formulating Countercheck trials.
Conclusion: Formulation (F4) containing 35 mg of Sodium Starch Glycolate & 40 mg of Crospovidone was found to be best one among all and also similar to the Marketed product (LAMICTAL-25) (f2= 73.17, f1= 3.65 & No significant difference, t= 0.0218) to marketed product.
Keywords: Lamotrigine; Super disintegrates; Crospovidone; Sodium starch glycolate; Sodium; Wetting time; Disintegration time; Non-Fickian diffusion
Abbreviations: ODT: Oral Disintegrating Tablet; SD: Solid Dispersion; PEG: Poly Ethylene Glycol; CP: Crospovidone; SSG: Sodium Starch Glycolate; X1: Amount of Crospovidone; X2: Amount of Sodium Starch Glycolate; mg: milligram; mL: milliliter; IPQC: In- Process Quality Control; % CDR: Percentage Cumulative Drug Release; RPM: Revolutions per minute; BCS: Biopharmaceutical Classification System; UR: Un Released; Min: Minute; ºC: Degree Centigrade; mm: millimeter; t50%: Time taken to release 50% drug from dosage form; t90%: Time taken to release 90% drug from dosage form; WT: Wetting time; DT: Disintegration time dissimilarity factor; f1: Dissimilarity factor; f2: Similarity factor
Introduction
Swallowing is troublesome issue in situations like Dysphagia,
specifically for both pediatrics and geriatrics. Oral disintegrating
tablets created a new benchmark in the pharmaceutical trade.
Porous tablets, rapimelts, Oro dispersible tablets, mouth-dissolving
tablets, Fast dissolving tablets, quick dissolving, melt-in mouth
tablets were used frequently in the place of ODT [1].
They rapidly disintegrate/dissolve in oral cavity within (<)
1min [2-4]. They were prepared by various techniques; formed
ODTs show differences in sensual characteristics such as mouth
feel, swallowability and taste. They also show variations in product
performance like mechanical strength of tablet, drug release,
bioavailability & stability. Manufacturing methods employed
for formulating ODTs include mass extrusion, spray drying,
cotton candy process, lyophilization, molding, compaction (wet
& dry granulation, direct compression), patented technologies
(Durasolv®, Orosolv®) [5-7].
Lamotrigine is an anti-epileptic drug and it is approved in
the united states for the treatment of partial seizures and bipolar
disease, belongs to class-II under BCS Classification and exhibit
oral Bioavailability of about 98%. It is available as immediate
release and sustained release formulations in the market with
different strengths such as 25mg, 50mg and 100 mg. In 2009 GSK
received FDA approval for extended release version of Lamotrigine
(Lamictal-XR) [8-14].
To design an optimized formulation with an appropriate
dissolution rate in a short time period with minimum heuristics is
critical for Formulation Scientist. Response surface methodology
(RSM) is used when only a few significant factors are involved
in experimental optimization. The technique requires less
experimentation and time, thus proving to be far more effective
and cost-effective than the conventional methods of formulating
dosage forms [3,9,12]. In the current research investigation the
direct compression method was utilized to formulate tablets, due
to numerous advantages such as simplest and cost effective tablet
production method [15].
Materials and Methods
Lamotrigine was a gift sample procured from Meditech Pharma Pvt Ltd, Hyderabad, India. Mannitol, Crospovidone, Sodium Starch Glycolate were procured from SD Fine Chemicals, Mumbai. Other excipients were procured from LobaChemie Ltd, Mumbai.
Formulation development
Preparation of lamotrigine solid dispersion: PEG 6000 was melted in a beaker on a water bath maintained at 50- 60 °C. Required amount (D:E in 1:1) of the drug was then added to molten PEG 6000 and mixed thoroughly for 5min. The molten mixture was cooled rapidly by placing it in an ice bath for about 5min and solidified. The hardened mixture was powdered, sieved through #80, packed and stored in desiccators for further processing [9-10].
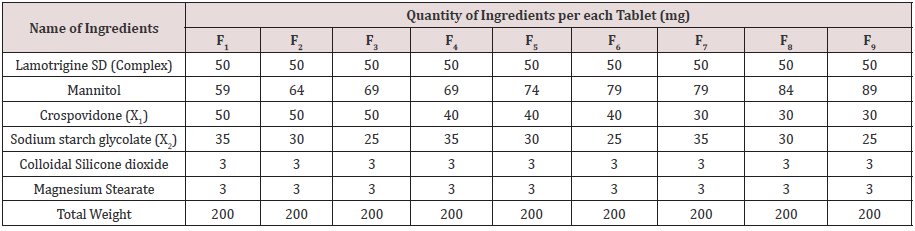
Preparation of lamotrigine fast dissolving tablets: Lamotrigine Tablets were prepared by direct compression method as per 32 factorial design. Experimental design was presented as Table 1. The formulae for the preparation of ODT were presented as Table 1. All ingredients were screened using #40 and mixed for obtaining uniform fine blends. Lubricants were screened through #80, mix them with above mixture and compressed to get ODT with the help of Tablet minipress (8 station) using 8mm circular punches. Obtained tablets were subjected to IPQC tests. Final tablets were transferred to airtight, light resistance containers for storage and further processing.
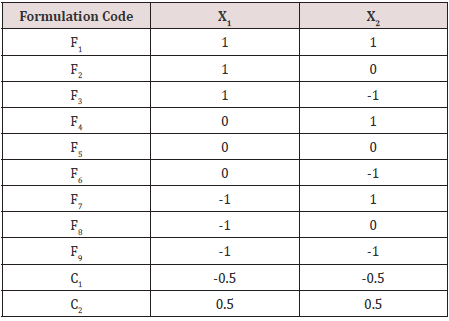
Where X1 means amount of Cospovidone and coded values as -1= 30 mg, 0=40mg, +1= 50mg.
X2 means amount of Sodium Starch Glycolate and coded values were-1= 25mg, 0=30mg, +1= 35mg.
Evaluation of Lamotrigine Fast Dissolving Tablets
Hardness
It was carried out with the help of Monsanto Tablet Hardness Tester [16].
Friability
The friability of the tablets was determined with the help of
Roche Friabilator. Weight of 20 Tablets noted as Initial weight (W0)
are dedusted in a drum for 4 min with a rotation rate of 25rpm and
weight was noted as Final weight (W). Percentage friability was
determined from following equation. The weight loss should not be
more than 1 % [16].
Friability (%) = (W0- W) / W0 x 100
Content uniformity
20 tablets were randomly selected, and the percent drug content was determined, the tablets contained not less than 92.5% or not more than 107.5% (100±7.5%) of the labeled drug content can be considered as the test was passed [9,17].
Assay
Select fixed number of sample on the random basis (20), comminute them to a obtain powder. The powder equivalent to 100mg Lamotrigine was weighed and transferred to 100mL volumetric flask containing 60mL of methanol and sonicated for 10min to solubilize the drug completely then dilute the methanolic solution with water to make up the final volume. From that prepare further dilution of 2mL aliquot in 100mL of 0.1 N HCl. The obtained solution was filtered through Whatman filter paper and absorbance of solution as measured at 254nm with the help of UV-Visible spectrophotometer [9].
Thickness
Thickness was determined with the help vernier calipers [18].
Wetting time
To measure Wetting time of the Tablet, a piece of Tissue paper folded twice was placed in a small petri dish (Internal Diameter is= 6.5cm) containing 5ml of Distilled water. A Tablet placed on the paper, and the time for complete wetting of the tablet was measured in sec [15-18].
In-vitro dissolution study
Lamotrigine oral disintegrating tablets subjected to dissolution test with the help of USP XXIII type-II tablet dissolution test apparatus using 900ml of 0.1N HCl operated under standard set of conditions. Withdraw samples at fixed intervals with the aid of syringe with a pre-filter and maintain the sink condition. Absorbances for samples were noted at 254nm using UV Visible spectrophotometer (after suitable dilutions if necessary). The determinations were performed in triplicate (n=3) [9].
Disintegration test
Disintegration of oral disintegrating tablets is achieved in the oral cavity owing to the impact of saliva, salivary volume is limited hence there is no proper In vitro In vivo correlation (IVIVC) was found in USP and IP. A modified method was used to determine to perform the disintegration test. A cylindrical vessel with 10 # was placed in such way that only 2ml of medium would be placed below the sieve. 6ml of medium was placed inside the vessel in such way that 4ml of the media was below the sieve and 2ml above the sieve. Tablet was placed on the sieve and the whole assembly was then placed on the top of agitator. Disintegration time was recorded. Six tablets were chosen randomly from the composite samples and the average value was determined [18].
Kinetic modeling of drug release
The dissolution profile of all the formulations was subjected to kinetic modeling [19-23].
Results and Discussion
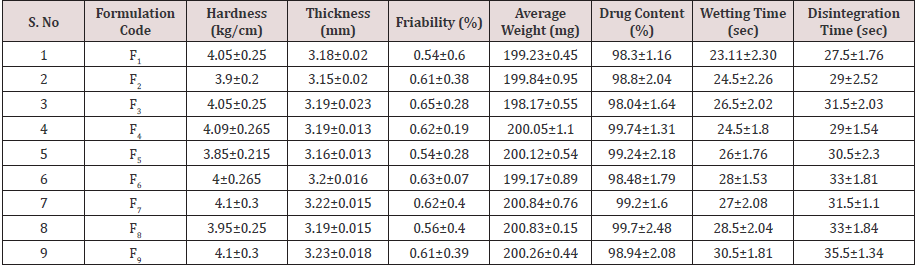
9 Lamotrigine Oral Disintegrating Tablet formulations were prepared by direct compression method using various proportions of super disintegrates combination as per 32 factorial design and the formulae presented in Table 1. All the formulations containing 25mg of Lamotrigine (as Lamotrigine SD with PEG as 1:1 ratio) prepared tablets prepared and evaluated for various pharmacopeial limits such as, drug content, mean hardness, friability, mean thickness, Weight variation as per official methods. Results shows that all the formulations were lie within the acceptance criterion; complete profile for finished product evaluation was presented as Table 2.
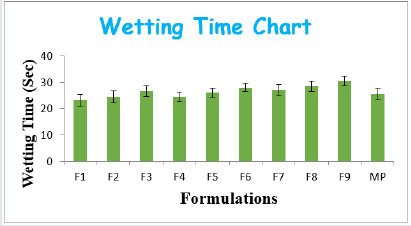
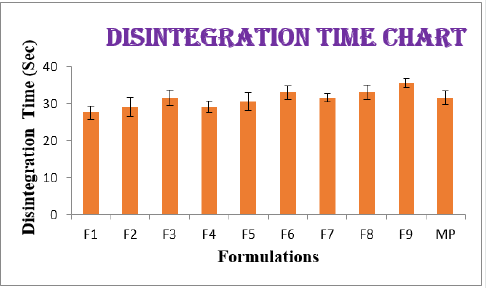
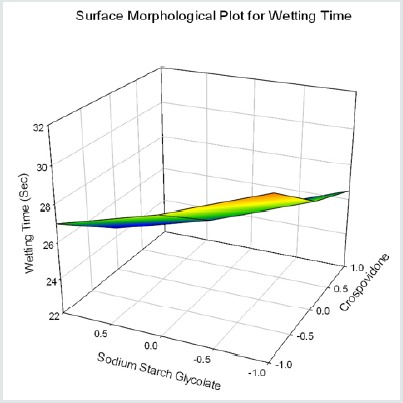
From the results of wetting time and Disintegration time, it reveals that as the concentration of super disinterants increases the wetting time decreases (Concentration of superdisintegrants inversely proportional to wetting time). Wetting time for all the formulations varied from 23.11±2.3 to 30.5±1.81sec. The Disintegration Time of tablets was in the range of 22.5±1.7- 35.5±1.34sec. Plots for wetting time and disintegration was presented as (Figure 1&2). The cumulative percentage drug released by each tablet in the In Vitro Release studies were based on the mean content of the drug present in the respective tablet.
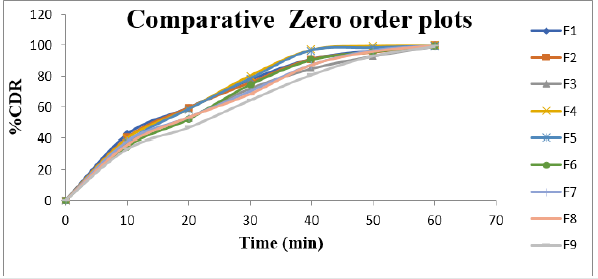
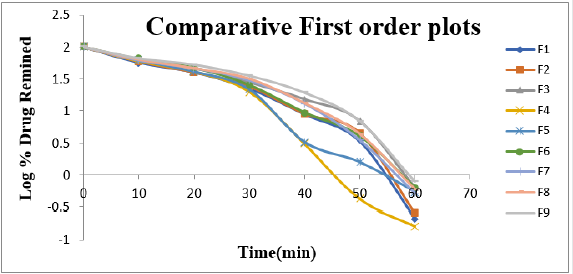
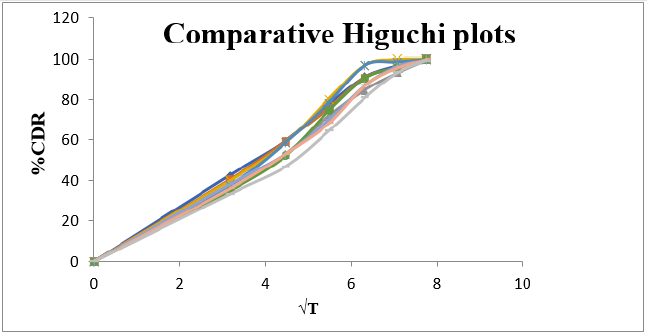
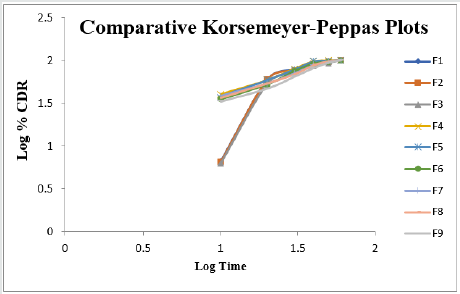
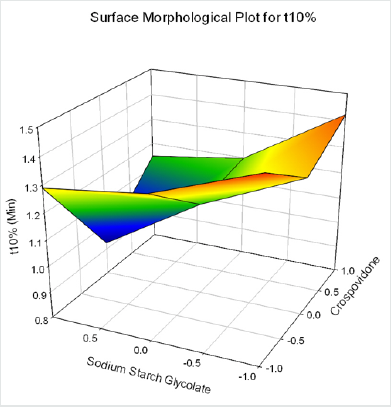
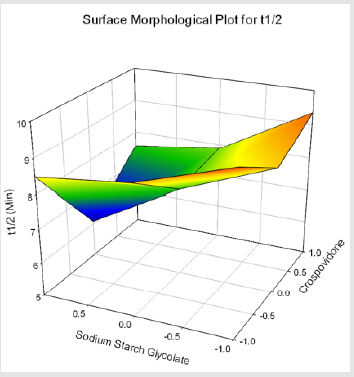
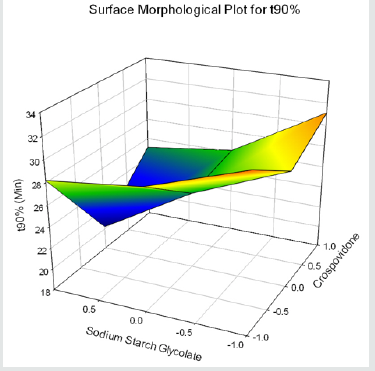
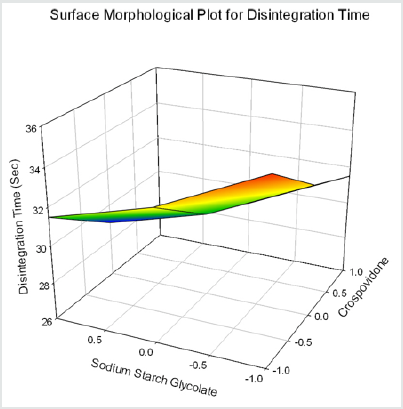
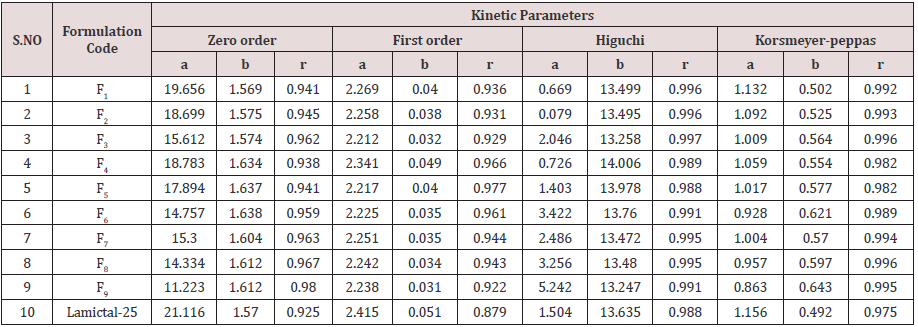
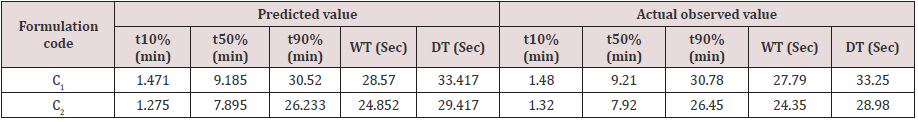
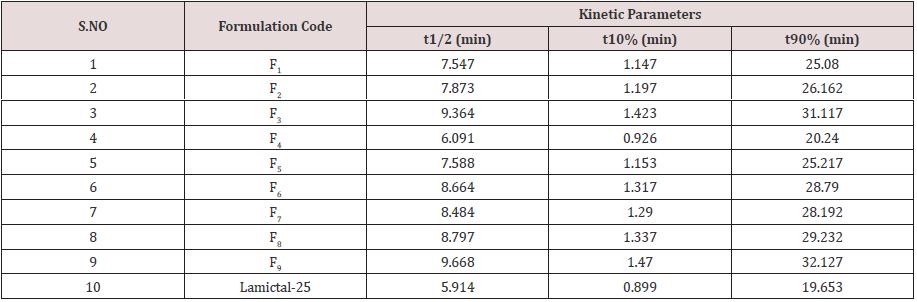
Cumulative % Drug release for F1-F9 at 60min was found to be in the range of 99.21±0.66-99.84±0.01%. Dissolution profiles of Lamotrigine ODT were subjected to goodness of fit test by linear regression analysis according to kinetic modeling to ascertain the drug release mechanism. The statistical parameters for kinetic models were determined and data present as Table 3 & 4 and plots represented as Figure 3-6 The values of r for formulations regarding Higuchi’s kinetics within a range of 0.988-0.997, Kinetic data also treated for Peppas equation, the slope (n) values ranges from 0.492-0.643 that shows Non-Fickian diffusion mechanism. Polynomial equations were developed for dependent variables such as t10%, t50%, t90%, Wetting time, Disintegration time. The validity of developed equations was verified by preparing counter check formulation (C1, C2). Results for counter check formulations presented Table 5. Response morphological plots shown in Figure 7-11.
Table 5:Dissolution/ Kinetic parameters.
Y1=1.251-0.055X1-0.141X2-0.024X1X2+0.178 X12+0.332X22 (for t10%)
Y2= 8.231-0.361X1-0.929X2-0.158X1X2+1.175 X12+0.217X22(for t50%)
Y3=27.351-1.199X1-3.087X2-0.525X1X2+3.903 X12+0.721X22(for t90%)
Y4=26.512-1.982X1-1.732X2+0.518 X12+0.268 X22(for Wetting Time)
Y5 =31.167-2X1-2 X2+0.5 X12+0.5 X22 (for Disintegration Time)
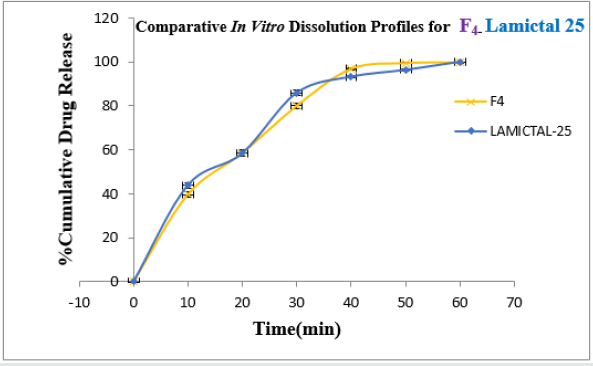
Formulation F4 containing 40mg of Crospovidone, 35mg of Sodium Starch Glycolate exerted promising dissolution parameter (Wetting time= 24.5±1.8sec, Disintegrating time = 29±1.54sec, t10% = 0.926min, t50% = 6.091min, t90% = 20.240min). The final best Formulation F4 is compared with Marketed product (LAMICTAL-25) tablets and Comparative Dissolution profiles shown in Figure 12. Which shows similarity (f2= 73.17, f1= 3.65).
Conclusion
The current research investigation focuses about influence of utilization of superdisinterants such as Crospovidone and Sodium Starch Glycolate in the formulation development of oral disintegrating tablet formulations of Lamotrigine. Results reveals that quantities of Superdisintegrant shows good impact on release of drug from formulation (directly proportional) The optimized formulation followed Higuchi’s kinetics while the drug release mechanism was found to be Non-Fickian Diffusion, first order release type. On the basis of evaluation parameters, the optimized formulation F4 may be used for the effective management of Epilepsy, convulsions. This may improve the patient compliance by showing rapid action via disintegration without difficult in swallowing and side effects which will ultimately improve the therapeutic outcome. We could be able to minimize the per oral cost of the Formulation (Table 6).
References
- K Kavitha, Kumutha Subramaniam, BoeyJiaHui, K Santhi, SA Dhanaraj, et al. (2013) Potential Drug Candidates for Fast Dissolving Drug Delivery-A Review. Research Journal of Pharmaceutical, Biological and Chemical Sciences 4(4): 1510-1526.
- Sehgal P, Gupta R, Umesh Kumar S, Chaturvedi A, Gulati A, et al. (2012) Fast Dissolving Tablets: A New Venture in Drug Delivery. American journal of PharmTech Research 2: 252-279.
- Raghavendra Kumar Gunda, JNSuresh Kumar (2016) Formulation Development and Evaluation of Risperidone Fast Dissolving Tablets.Journal of Pharmacy Research 10(9): 579-588.
- David E, Armen HT, Ethrin JA, April W Armstrong (2008) Principles of pharmacology. (2nd), The pathophysiologic basis of drug therapy. Wolters Kluwer (India) Pvt. Ltd, New Delhi, India pp. 815.
- Rajeev soni, Galividyasagar (2013) Design and development of quick dissolving tablet Containing loratadine by direct compression method. International Journal of Pharmaceutical, Chemical and Biological Sciences 3 (3): 771-800.
- Gupta A, Mishra AK, Gupta V, Bansal P, Singh R, et al. (2010) Recent Trends of Fast Dissolving Tablet - An Overviewof Formulation Technology. International Journal of Pharmaceutical and Biological archives p. 1-10.
- Manivannan R (2009) Oral Disintegrating Tablets: A Future Compaction. International Journal of Pharma Research and Development 1(1): 1-10.
- Lalic M, Pilipovic A, Golocorbin-Kon S, Gebauer-Bukurov K, Bozic K, et al. (2011) Comparison of Dissolution Profiles and Serum Concentrations of two Lamotrigine tablet formulations. Drugs RD 11(1)
: 53-60. - Gunda RK, Kumar JNS, Babu CA, Anjaneyulu MV (2015) Formulation Development and Evaluation of Lamotrigine Sustained Release Tablets Using 32 Factorial Design. International Journal of Pharmaceutical Sciences and Research 6(4): 1746-1752.
- Koteswari P, Sunium S, Srinivasababu P, Babu GK, Nithya PD (2014) Formulation Development and evaluation of fast disintegrating tablets of Lamotrigine using liquid-solid technique. Int J Pharm Investig 4 (4): 207–214.
- Madhuri T Hivarkar, Ravi D Hole (2018) Formulation and Evaluation of Fast Dissolving Tablet of Lamotrigine. International journal for Pharmaceutical Research Scholars 7: 50-57.
- PK Lakshmi, Swetha Reddy, C Kishore, B Satish Reddy (2013) Formulation and Evaluation of Oral Disintegrating Tablets of Lamotrigine Solid Dispersions. Iranian Journal of Pharmaceutical Sciences 7: 1-12.
- Mohan A, Gundamaraju R (2015) In vitro and In vivo evaluation of fast-dissolving tablets containing solid dispersion of Lamotrigine. Int J Pharm Investig 5(1): 57-64.
- Jatinderpal Singh, Rajeev Garg, Ghanshyam Das Gupta (2015) Enhancement of Solubility of Lamotrigine by Solid Dispersion and Development of Orally Disintegrating Tablets Using 32 full Factorial Design. Journal of Pharmaceutics 1-8.
- Raghavendra Kumar Gunda, Prasada Rao Manchineni, Chaikam Gopi Reddy, Kurapati Divya, Duddu Alekhya (2019) Formulation development and in vitro evaluation of Levetiracetam oral disintegrating tablets. Mintage Journal of Pharmaceutical and Medical Sciences 8: 1-6.
- Gunda Raghavendra Kumar, JN Suresh Kumar, V Satyanarayana, G Swarupa Rani, B Satya Prasad (2016) Formulation Development and Evaluation of Clopidogrel Fast Dissolving Tablets. Iranian Journal of Pharmaceutical Sciences 12(2): 61-74.
- Raghavendra Kumar Gunda, Jujjuru Naga Suresh Kumar (2017) Formulation Development and Evaluation of Moxifloxacin Hcl Fast Dissolving Tablets. Pharmaceutical Methods 8: 160-167.
- Raghavendra Kumar Gunda, JN Suresh Kumar, V Satyanarayana, Swathi Batta, Ch Meher Harika (2016) Formulation Development and Evaluation of Carbamazepine Fast Dissolving Tablets. Journal of Pharmacy Research 10: 216-225.
- Raghavendra Kumar Gunda, A Vijayalakshmi (2019) Formulation Development and Evaluation of Gastro retentive Bio adhesive drug delivery system for Moxifloxacin.HCl. Indian Journal of Pharmaceutical Education and Research53(4): 724-732.
- Notari RE (1987) Biopharmaceutics and clinical pharmacokinetics. (4th ), New York: Marcel Dekker Inc, USA p. 6-21.
- Higuchi T (1963) Mechanism of sustained-action medication Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. Journal of Pharmaceutical Sciences 52: 1145-1149.
- Raghavendra Kumar Gunda, Jujjuru Naga Suresh Kumar (2018) Formulation Development and Evaluation of Amisulpride Fast Dissolving Tablets. FABAD Journal of Pharmaceutical Sciences 43(2): 105-115.
- Peppas NA (1985) Analysis of fickian and non-fickian drug release from polymers. Pharm Acta Helv 60(4): 110-111.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...