Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Research Article(ISSN: 2637-4706) 
Comparison and Evaluation of Freely Supplied Government and Ethically Marketed Antihypertensive Drug (Atenolol) Volume 3 - Issue 3
Injamamul Haque*, Shubajit Ghosh, K Hanumanthachar Joshi, Neethu M Saji, and Md Jesarul SK
- Pharmaceutics Department, SVCP Mysore, India
Received: March 09, 2020; Published: March 17, 2020
Corresponding author:Injamamul Haque, Pharmaceutics Department, SVCP Mysore, India
DOI: 10.32474/DDIPIJ.2020.03.000164
Abstract
The main aim and objective of present research work is to evaluate and compare the standards concerning quality of generic and two branded antihypertensive drug (atenolol). The drugs are evaluated and results showed that branded and generic meet the pharmacopeial specifications. All tablets passed for the test of weight variation, hardness, thickness, friability, disintegration, dissolution, as per pharmacopoeia. Hence, we can say that branded and non-branded drugs of antihypertensive are equal. So, health care professionals are suggested to prescribe generic drugs so that everyone can reach the coast of drugs and maintain health.
Keywords: Atenolol; In vitro studies; Physicochemical test
Introduction
In the present framework medical stream has converted into a curative jungle. Pharmaceutical industries are out coming with many new molecules to cure many terrible diseases. The single comprehensive drug is formulating by various pharmaceutical companies by different brand names. In various research studies it is found that especially in rural areas most of the drugs are devour without proper prescription, which is very dangerous activity followed, apart from these few drugs are selling by government as the OTC drugs to treat common diseases. It is found that few smallscale Pharma industries are also following the Pharmacopeial standards which are maintained by pharmaceutical regulatory authorities during the formulation of the drugs. The same generic drug is manufactured by different pharmaceutical companies under different brand names and sold under different coast. The present studies aim to throw away the blind belief of many people that branded drugs show better therapeutic activity than the generic drugs. Hence quality of drugs accessed by qualitative and quantitative analysis of the drug formulation. As per pharmaceutical standards the parameters like weight variation, hardness, friability, disintegration, dissolution and pH of the drug are studied. In the present study we are analyzing antihypertensive drug (atenolol) used for treating hypertension [1-7].
Materials and Methods
Chemicals and reagents
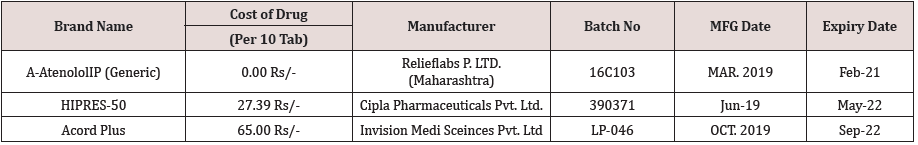
The atenolol tablets were taken from one of the reputed pharmaceutical stores as well as taken from the Government hospital pharmaceutical stores (Table 1).
Methodology
Various analytical methods and tests are important for the development and manufacture of pharmaceutical formulations. The evaluation was done according to USP and BP standards.
Evaluation Tests for Tablets
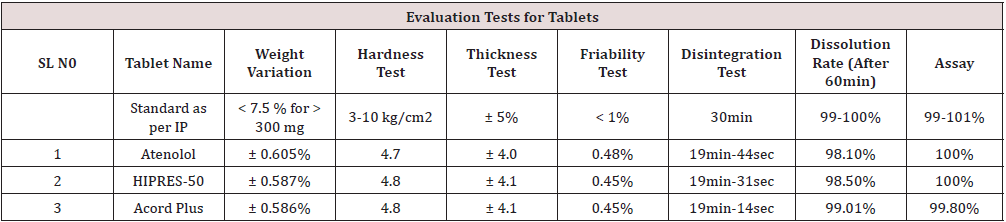
Weight variation
Weigh Sample tablets (20) of each brand individually on electronic analytical balance the average weight and the percentage (%) deviation was determined [8-15].
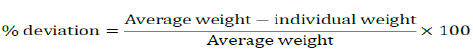
Limits-As per I.P generally 10%for tablet weighing is 120mg (or) less than 7.5% for tablet weighing more than 300mg. The results are shown in Table 2.
Hardness test
The hardness of 5 atenolol tablets of each brand was measured by using Monsanto hardness tester. The hardness determines the resistance of tablet for breakage, under conditions of storage, transportation and handling, before usage. Limits-As per I.P 3-10kg.
Thickness test
The thickness of tablet is determined by measuring the diameter of tablet. It is a major quality control test which helps in packaging. The excessive thickness of tablet cause problems during blister or plastic container packaging. Limits-Diameter, % Deviation, greater than 12.5 ± 3%, Less than 12.5 ± 5%.
Friability test
Friability determines the physical strength of tablet upon exposure to mechanical shock and attrition. Roche friabilator was used to measure the friability. The initial weight of 20 tablets was taken as (W1) and placed in a friabilator for 4 min at a rate of 25rpm for 100. After 100 revolutions the tablets were weighed again and noted as (W2). The difference between the weight before and after the process is determined as Friability and should not exceed 1%. The percent friability was determined using the following formula.
% Friability = [(Initial weight (W1) – Final weight (W2))/Initial weight (W1)] ×100
Limits-As per I.P friability limits are does not cross the more than 1%.
Disintegration test
The disintegration time was performed by apparatus specified in USP at 50rpm. 900ml of buffer pH 7.4 was used as disintegration medium and the temperature of 37± 0.5 0C and time in seconds was taken for complete disintegration of the tablet.
Dissolution studies
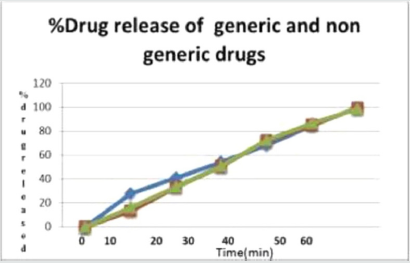
Drug dissolution testing is routinely used to provide critical in vitro drug release information for both quality control purposes. The Atenolol tablets are added to 900ml of dissolution media (PH 7.4 phosphate buffer) contained in USP dissolution apparatus II and stirred at a speed of 60rpm at 37 ± 0.5 °C. Ten milliliter aliquots were withdrawn at interval of 10, 20, 30, 40, 50, 60 minute and replaced by 10ml of fresh dissolution media (37 °C). The samples were collected and analyzed after suitable dilution at 252nm using Shimadzu 1700 UV-Visible spectrophotometer (Figure 1).
Assay
The spectroscopic method (UV-visible) is chosen for ascertaining the percentage purity of samples they are
UV Method
Preparation of standard stock solution
The standard solution of Atenolol is prepared by dissolving the weighed 10mg of drug sample into a 100ml volumetric flask and the volume is make up to 100ml to get the concentration of 100μg/ ml using methanol as a solvent.
Preparation of calibration curve and λmax of Atenolol determination
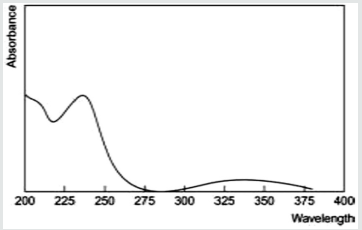
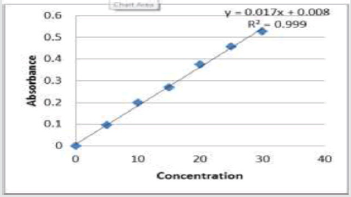
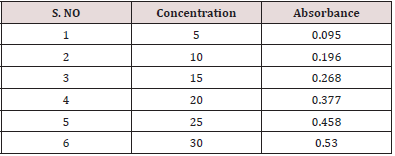
From the above prepared standard stock solution fresh aliquots were pipette out and suitably diluted with methanol to get final concentration in the range of 5-30μg/ml. The prepared dilutions were scanned under 200-400nm wavelength range and a sharp peak was obtained at 241nm (Figure 2). Calibration curve was plotted by taking concentration of solution on x-axis and absorbance on yaxis. The λmax is shown in (Figure 3).
Procedure
Weigh about 20 tablets of Atenolol, powdered and weigh equivalent amount of drug about 10mg and transferred into a 100ml volumetric flask. Then add few ml of methanol and make it to dissolve and the final volume were made up to mark with methanol. The solution was then filtered through Whatman filter paper and the absorbance was measured against blank. The amount of atenolol was computed by using the equation referring to the calibration curve data represented in (Table 3).
Results and Discussion
The results of our research work conducted on generic and two different brands of antihypertensive atenolol tablets, met the IP requirements of quality control tests within specified limits. It is carried out in an in vitro study. The various physical parameters of tablets like weight variation, hardness, thickness, friability, dissolution, assay and disintegration time are accessed were within the pharmacopeial specifications. Disintegration time of the entire branded and generic tablet was found in the pharmacopeial limit while generic tablet showing little higher disintegration. Drug release of generic tablet was found to be 99.1 % in 30 minutes which is comparatively lesser than the branded tablets which showed drug release 100% in 30 minutes. Hence, it can be concluded that tablets were all found to be as per pharmaceutical specifications.
Conclusion
Finally, study suggests that generic and branded (non-generic drugs) shown equal results. Hence generic form of the drug should be widely prescribed to reduce the medication cost and make the treatment economical. So that general people can also meet the medication cost.
Acknowledgement
The authors are thankful to Management, Principal and Staff members of Sarada Vilas College of Pharmacy, Mysuru for providing necessary facilities.
References
- Indian Pharmacopoeia (I.P.).
- Martindale (1996) The Extra Pharmacopoeia. Reynolds J E F pp. 863-864.
- Narkiewicz K, Somers VK (1999) Obstructive sleep apnea as a cause of neurogenic hypertension. Curr Hypertens Rep 1(3): 268-273.
- DA Skoog, DM West, FJ Holler (1994) Analytical Chemistry an Introduction. p. 1-3.
- RD Smith, AT Chiu, PC Wong, WF Herblin, PBMWM Timmermans Annu (1992) Pharmacology of Nonpeptide Angiotensin II Receptor Antagonists. Rev Pharmacol Toxicol 32: 135-165.
- Chiu AT, McCall DE, Price WA, Wong PC, Carini DJ, et al. (1990) Nonpeptide angiotensin II receptor antagonists. VII. Cellular and biochemical pharmacology of DuP 753, an orally active antihypertensive agent. J Pharmaco ExpTher 252(2): 711-718.
- Williams RC, Alasandro MS, Fasone VL, Boucher RJ, Edwards JF (2002) Comparison of liquid chromatography, capillary electrophoresis and super-critical fluid chromatography in the determination of Losartan Potassium drug substance in Cozaar tablets. J Pharm Biomed Anal 14(11): 1539-1546.
- Prabhakar AH, Giridhar R (2002) A rapid colorimetric method for the determination of Losartan potassium in bulk and in synthetic mixture for solid dosage form. J Pharm Biomed Anal 27(6): 861-866.
- Zhao Z, Wang Q, Tsai EW, Qin XZ (1999) Identification of losartan degradates in stressed tablets by LC-MS and LC-MS/MS. J Pharm Biomed Anal 20(2): 129-136.
- McCarthy KE, Wang Q, Tsai EW, Gilbert RE, Ip DP, et al. (1998) Determination of losartan and its degradates in COZAAR tablets by reversed-phase high-performance thin-layer chromatography. J Pharm Biomed Anal 17(5): 671-677.
- Farthing D, Sica D, Fakhry I, Pedro A, Gehr TW (1997) Simple high-performance liquid chromatographic method for determination of losartan and E-3174 metabolite in human plasma, urine and dialysate. J Chromatogr B Biomed Sci Appl 704(1-2): 374-378.
- Ritter MA, Furtek CI, Lo MW (1997) An improved method for the simultaneous determination of losartan and its major metabolite, EXP3174, in human plasma and urine by high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal 15(7): 1021-1029.
- Pawlak Z, Clark BJ (1992) The assay and resolution of the beta-blocker atenolol from its related impurities in a tablet pharmaceutical dosage form. J Pharm Biomed Anal 10(5): 329-334.
- S Bhimana, GS Guntuku (2017) Journal of Global Trends in Pharmaceutical Sciences. 8(1): 3599-3608.
- P Swathi, C Rajesekhar (2016) Int. J Res Pharm Sci 1: 39-42.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...