Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Research Article(ISSN: 2637-4706) 
A Review on Biomedical Applications of Polymeric Nanoparticles Volume 2 - Issue 3
A Krishna Sailaja*
- Associate Professor, RBVRR Women’s college of pharmacy, Osmania University, India
Received: September 22, 2018; Published: October 03, 2018
Corresponding author: A Krishna Sailaja, Associate Professor, RBVRR Women’s college of pharmacy, affiliated to Osmania University, Hyderabad, India
DOI: 10.32474/DDIPIJ.2018.02.000140
Abstract
Splendid achievements have been made in management of disease through invention of drugs over past decade. The present conventional drug delivery systems often have side-effects and complications due to their wide distribution throughout the body fluids. The localization of drug action in injured tissue is a promising way to solve this problem. The objective of drug targeting is to achieve a desired pharmacological response at a selected site without undesirable interaction at other sites. This is especially important in cancer chemotherapy and rheumatoid arthritis treatment. Recently invention of drugs has been generated by various drug delivery systems like microspheres, liposomes, noisomes, nanocapsules and nanoparticles. Among all colloidal drug carriers’ nanoparticles are gaining more popularity because of their stability, easy preparation, for achieving reduced toxicity, for increased drug efficacy & site targeted action. Nanoparticulation is a very useful strategy towards targeted drug delivery and also for enhancement of bioavailability of low soluble drugs. In this article nanoparticles preparation methods and their biomedical applications were discussed in detail.
Keywords: Nanoparticles; Polymeric Materials; Bottom Up Approach
Introduction
Nanotechnology has achieved breakthrough in therapeutics, bioengineering, diagnostics, imaging, and optics in recent vintage [1]. The development of nanosystems by tailoring the macromolecules is the recent topic of interest. As nanoparticles possess extraordinary, often tunable properties dramatically different from the bulk materials, such as high surface to volume ratio, particle size and so forth there is an enormous demand for the tailor-made functional nanoparticle systems. Inorganic, organic or hybrid nanoparticular materials are used in various applications fields as medicine, pharmaceuticals, analytics, catalysis, coating, and several others. Nanoparticles are efficient and versatile devices for drug delivery as they can improve crucial properties of a drug entity such as solubility, pharmacokinetic, biodistribution and in vivo stability [2]. Due to their tailoring properties they can overcome physiological barriers and can help to guide their payload to specific cells or intercellular compartments. By which side effects can be minimized and therapeutic benefits of a drug can be increased. By virtue of their small size and by functionalizing their surface with polymers and appropriate ligands, polymeric nanoparticles can also be targeted to specific cells and locations in the body. Depending on the polymer characteristics, polymeric nanocarriers can also be engineered in such a way that they can be activated by changes in the environmental pH, chemical stimuli, or temperature. Macrophages are well recognized phagocytic cells of the reticuloendothelial system (RES) and one of the main cells responsible for the uptake and clearance of administered drug loaded nanoparticles. In general, once nanoparticles are opsonised, endocytosis/phagocytosis occurs, and the nanoparticles are incorporated in an endolysosome/phagolysosome and degrade [3]. However, the ability of various nanoparticles to escape the endolysomal compartment allows incorporated drugs to be delivered to the cytoplasm and finally to the nucleus. Thus, this property of the nanoparticles to be easily taken up by phagocytic cells makes them feasible to carry proteins, genes and other biological macromolecules as well [4]. Other applications include cytoplasmic release of plasmid vectors and therapeutic agents (e.g. for cytoplasmic infections and for slow cytoplasmic release of drugs that act on nuclear receptors) [5]. Depending on the preparation methods used, two different types of nanoparticles can be obtained, namely nanospheres and nanocapsules [6,7]. Nanoparticles are drug loaded particles with diameter ranging from 1 to 1000nm.
Necessity of Nanoparticulate Drug Delivery Systems [8-10]
Controlled drug delivery systems are those type of devices in which therapeutic agents may be released at controlled rates for long periods of time, ranging from days to months. The control exercised over the nature of drug delivery may be in temporal nature (rate controlled) or of a spatial nature (site controlled) or both. Currently there are a limited number of formulations approaches available for the compounds that are soluble in water, that includes, solubilisation, cosolvency, complexation with beta-cyclodextrines and solid dispersions that can enhance the dissolution of the drugs. Another classical formulation approach for poorly soluble drugs is micronisation, that means the transfer of coarse drug powder into ultrafine powder. It’s a technology for BCS classified class II drugs that are having a good permeability but a low bioavailability due to their poor solubility. But for the colonic drug delivery, micronisation often results in a low and variable bioavailability. Hence, the next step taken to improve the saturation solubility, dissolution velocity and bioavailability of drugs is by reducing the particle size from microns to nano size levels that can be termed as Nanonisation. Hence, it was thought that nanoparticle could be used as an ideal drug delivery.
The major goals in designing nanoparticles as a delivery system are to control particle size, surface properties and release of pharmacologically active agents in order to achieve the sitespecific action of the drug at the therapeutically optimal rate and dose regimen. Though liposomes have been used as potential carriers with unique advantages including protecting drugs from degradation, targeting to site of action and reduction toxicity or side effects, their applications are limited due to inherent problems such as low encapsulation efficiency, rapid leakage of water-soluble drug in the presence of blood components and poor storage stability. On the other hand, polymeric nanoparticles offer some specific advantages over liposomes. For instance, they help to increase the stability of drugs/proteins and possess useful controlled release properties. Nanoparticles have a relatively large surface which promotes its ability to bind, adsorb and carry other compounds such as drugs and proteins. Although the definition identifies nanoparticles as having dimensions below 0.1 micro meter and 100nm, especially in the area of the drug delivery relatively large sized nanoparticles (greater than 100nm) may be needed for loading sufficient amount of drug onto the particles.
Method of Synthesis
Nanomaterials exhibit unique properties at nanoscale of 1 to 100 nanometer (nm). However, achieving sizes <100nm is more feasible with hard materials (like Silica, Metal oxides and diamonds) compared to drug and polymer molecules, which are soft materials. Production of nanoparticles for drugs that are usually soft materials with melting point below 300⁰C is much more challenging than that of hard materials because of high stickiness of the former. For this reason, it is a reasonable goal to aim at <300nm for drug and polymer materials. Hence two basic approaches are employed for the synthesis of nanostructures in the 50-300nm range for drug delivery, irrespective of the field or discipline. The two approaches are ‘Bottom-Up’ approach and ‘Top-Down’ approach [2].
‘Bottom-Up’ Approach
The building of nanostructures is achieved by growing or assembling of atoms or molecules which are the building blocks. The building blocks may be manipulated through controlled chemical reactions to self-assemble and make nanostructures such as nanotubes and quantum dots. Atoms or molecules may also be physically manipulated to form nanostructures using minute probes. Self-assembling of atoms or molecules can be achieved by templating and non-templating. Templating involves the interaction of bio macromolecules under the influence of a specific sequence, pattern, structure, external force or spatial constraint. Non-templating is the formation of nanostructures from atoms or molecules with external influence.
‘Top-Down’ Approach
Bulk materials are reduced by some processes to form nanostructures. ‘Top down’ is achieved by breaking or etching techniques which is achieved by bulk machining, surface machining and mold machining employing lithography.
Bio Medical Applications of Nanoparticle [11-13]
Medicine: The biological and medical research communities have exploited the unique properties of nanomaterials for various applications (e.g., contrast agents for cell imaging and therapeutics for treating cancer). Terms such as biomedical nanotechnology, bio nanotechnology, and nanomedicine are used to describe this hybrid field. Functionalities can be added to nanomaterials by interfacing them with biological molecules or structures. The size of nanomaterials is similar to that of most biological molecules and structures; therefore, nanomaterials can be useful for both in vivo and in vitro biomedical research and applications. Thus far, the integration of nanomaterials with biology has led to the development of diagnostic devices, contrast agents, analytical tools, physical therapy applications, and drug-delivery vehicles.
Diagnostics: Magnetic nanoparticles bound to a suitable antibody are used to label specific molecules, structures or microorganisms. Gold nanoparticles tagged with short segments of DNA can be used for detection of genetic sequence in a sample. Multicolor optical coding for biological assays has been achieved by embedding different-sized quantum dots, into polymeric micro beads. Nanopore technology for analysis of nucleic acids converts strings of nucleotides directly into electronic signatures.
Drug Delivery: The overall drug consumption and sideeffects can be lowered significantly by depositing the active agent in the morbid region only and in no higher dose than needed. This highly selective approach reduces costs and human suffering. An example can be found in dendrimers and nanoporous materials. They could hold small drug molecules transporting them to the desired location. Some potentially important applications include cancer treatment with iron nanoparticles or gold shells. A targeted or personalized medicine reduces the drug consumption and treatment expenses resulting in an overall societal benefit by reducing the costs to the public health system.
Delivery of Anticancer Drugs: Polyalkyl cyanoacrylate nanoparticles have been studied for targeting drugs to specific sites in the body. The small colloidal carriers are biodegradable and drug substances can be incorporated by a process of surface adsorption
Eg: Doxorubicin associated with polyisohexylcyanoacrylate nanoparticles, Mitoxantrone in Polybutylcyanoacrylate nanoparticles, aclacinomycinA in polyisobutylcyanoacrylate nanoparticles, acyclovir in Polybutylcyanoacrylate nanoparticles and doxorubicin in polyalkyl cyanoacrylate nanoparticles, granulocyte colony stimulating factor in polyalkyl cyanoacrylate nanoparticles.
Nanoparticles Can Be Used for Targeting Inflammatory Bowel Disease: ALF Lanprecht, Nathalieubrich etal studied the use of nanoparticles for targeted oral drug delivery to the inflamed gut tissue in inflammatory bowel disease. Local drug delivery would be a distinct improvement compared with existing colon delivery devices for this disease. Rolipram is an anti-inflammatory model drug was incorporated within poly(lacticcoglycolic acid) nanoparticles which were administered once a day orally for a five consecutive days. A clinical activity score and myeloperoixidase activity were determined to assess the inflammation.
Nanoparticles for Per Oral Administration of Proteins and Peptides
Proteins and peptides are seen as therapeutic drugs. They are susceptible to proteolytic degradation. Therefore, lead to problems of physiochemical and bio-stability coupled with their short halflife and inability to pass most of the biological barriers. Polyalkyl cyanoacrylate nanoparticles and nanocapsules were developed as peptide carriers for insulin and growth hormone releasing factor. PACA nanoparticles were developed as drug delivery system for cyclosporine.
Biodegradable Polymeric Nanoparticles as Protein Carrier: Nanoparticles, first developed around 1970, are polymeric particles ranging in size from 10 to 1000nm. They were initially devised as carriers for vaccines and anticancer drugs. Polymeric nanoparticles with biodegradable and biocompatible polymers are good candidates for peptide drug delivery, and there has been considerable interest in the use of nanoparticles (NP) as potential protein delivery systems.
Nanoparticles for Intra-Arterial Application: They are used for the intra-arterial localization of therapeutic agents. Advantages include their sub cellular size, targeted surface, good susceptibility, uniform dispersity for catheter-based therapy and an easy penetration into the arterial wall without causing trauma. Labhasetwar et al. 1995 demonstrated that local delivery of drugs like Dexamethasone, heparin and U-86983 was facilitated and high regional concentration could be built with prolonged retention in lower doses with reduced systemic toxicity.
Nanoparticles for Brain Delivery: The blood brain barrier represents one of the hurdles for drugs including antibiotics, antineoplasic agents. One of the possibilities to overcome this barrier is drug delivery to brain using nanoparticles. Drugs used for brain targeting include hex peptide dalargin, the dipeptide kyotropin, loperamide, tubocurarine and doxorubicin. Kreuter and co-workers in a series of studies advocated nanoparticles for brain delivery. They reported transport of the hex peptide dalargin across the blood brain barrier using poly(butyl cyanoacrylate) nanoparticles which were coated with polysorbate 80. Some neuropeptides are also delivered across blood-brain barrier using nanoparticle technology.
Work Done on The Nanoparticle Drug Delivery System
Hasaan A et al. [14] aimed to prepare anti-glaucomatous Dorzolamide hydrochloride-(Dorzo) loaded nanoparticles as a controlled release system. Eudragit RS 100 (RS) and/or RL 100 (RL) were used in formulations by an opportunely adapted Quasiemulsion solvent diffusion technique. The formulations were evaluated in terms of particle size, zeta potential, drug entrapment, and release profile. All formulations showed tiny particle size varying from 114 to 395 nm for RS and 65 to 277 nm for RL. Positive zeta potential was +19 to +32 mV for RS and +23 to +42 mV for RL formulations. It was demonstrated that increasing polymer concentration lead to increase the percentage of drug entrapped in all batches, to a certain extent (drug: polymer 1:4). Nanoparticles prepared using RL showed lower entrapment efficiency than RS. A prolonged drug release was shown by all the formulations. Increasing the polymer concentration caused a decrease in the release rate. M Bharath et al. [15] prepared and investigated the Valsartan nanoparticles by nanoprecipitation method and the various formulations were prepared by optimizing. The prepared nanoparticles were characterized by FTIR, DSC, SEM, particle size analysis, In vitro diffusion and in vivo studies are been performed. The particle sizes of the prepared nanoparticles were ranging from 175 to 232 nm. From three formulations, F2 formulation showed best release of 60.38 % at the end of 24th h. In vivo studies revealed that in case of free drug,40.9mcg/ml drug of maximum dose was recovered but in case of nanoparticles the dose recovered in serum was 16.02 mcg/ml after 6hr.
S Tamizharsi et al. [16] aimed to prepare and evaluate polymethacrylic acid nanoparticles containing Lamivudine in different drug to polymer ratio by nanoprecipitation method. SEM indicated that nanoparticles have a discrete spherical structure without aggregation. The average particle size was found to be 121nm. The particle size of the nanoparticles was gradually increased with increase in the proportion of polymethacrylic acid polymer. The drug content of the nanoparticles was increasing on increasing polymer concentration up to a particular concentration. No appreciable difference was observed in the extent of degradation of product during 60 days in which, nanoparticles were stored at various temperatures. The in-vitro release behavior from all the drug loaded batches was found to be zero order and provided sustained release over a period of 24 h. The developed formulation overcome and alleviates the drawbacks and limitations of Lamivudine sustained release formulations and could possibility be advantageous in terms of increased bioavailability of Lamivudine. Anbarasan B et al. [17] aimed to study and optimize the formulation and the In-vitro evaluation of Chloroquine Phosphate loaded Chitosan Nanoparticles. The drug content of Nanoparticles increased on increasing the polymer concentration up to a particular level. Entrapment efficiency of 92.87% was achieved with drug to polymer ratio 1:6. In-vitro release of Chloroquine Phosphate from Chitosan Nanoparticles was 85.13% within 24 h. Good stability was observed at refrigeration condition compared to other temperature conditions during eight weeks of storage.
Paresh N Patel et al. [18] formulated and evaluated chitosan nanoparticles of Tamoxifen citrate for cancer therapy. Nanoparticles of TMX were prepared using chitosan using ionic gelation method. The concentration of the polymers Chitosan was selected based on the results on preliminary screening. The nanoparticles prepared were evaluated for morphology, loading efficiency, Invitro release and Invitro anticancer activities. The formulations FM-1 showed good drug release from the polymer. The percentage cumulative drug release after 3,4,5,6,7 and 8 hours was 40.54, 48.68, 56.26, 65.84, 71.42 and 78.03% respectively. Among the all formulations FM 1 showed maximum drug release in 8 hours diffusion study and good entrapment efficiency. Jaimin D Patel et al. [19] prepared and evaluated methotrexate loaded ethyl cellulose nanoparticles by solvent evaporation method with PVA as dispersion medium. Nine batches of nanoparticles were optimized by varying drug: polymer ratio and dispersion medium were prepared by using 32 factorial designs. Prepared nanoparticles were evaluated with respect to particle size, drug entrapment efficiency and in vitro release study. The results for the best formulation showed a mean particle size of 267nm, entrapment efficiency 65.07 % and in-vitro release 96.97 %. In-vitro release pattern of methotrexate from nanoparticles in pH 7.4 phosphate buffer showed a biphasic pattern with an initial burst effect and prolonged release over 24hrs.Stability studies showed that similar drug content and closest in vitro release profile to initial data when the sample stored at 25⁰C.
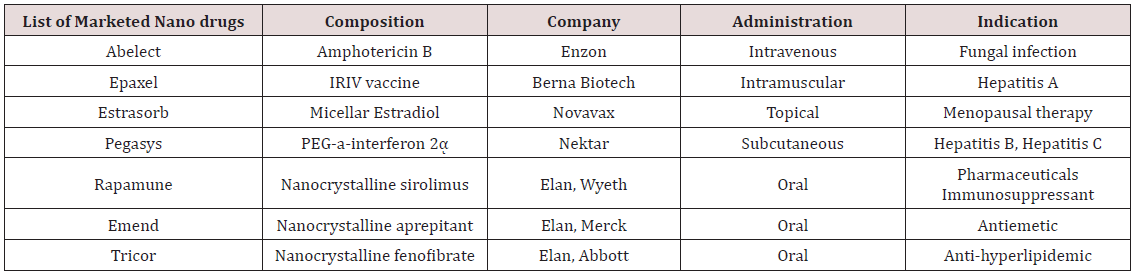
Poovi et al. [20,21] formulated and optimized Repaglinide (RG) loaded chitosan (CN) nanoparticles were prepared by solvent evaporation method in three different ratios. The prepared nanoparticles were characterised for particle size, FTIR, percentage yield, drug entrapment and for invitro release kinetics. The surface morphology of these RG-CN preparations was found to be smooth. At high polymer concentration RG-CN preparations showed high drug loading and encapsulation efficiency with nano size. Invitro release kinetics studies shown that RG loaded CN nanoparticles were capable of releasing the drug in a slow sustained manner for 15 days following first order release with fickian diffusion. From their investigation it was concluded that the repaglinide loaded chitosan nanoparticles is an effective carrier for the design of a controlled drug delivery system (Table 1).
Conclusion
Nanoparticle drug delivery system was found to be very important drug delivery system for targeting of anti-neoplastic agents, anti-rheumatic agents, vaccines and genes. Scientist can target brain, lungs, eye and arteries more effectively by applying nanotechnology principles. Toxicity caused by these nanoparticles is now becoming a great problem. Measurements should be taken to avoid toxicity generated by the nanoparticles.
References
- Anil Mahapatro, Dinesh K Singh (2011) Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. Journal of bio technology 9: 55.
- Manivannan Rangasamy, Kugalur Ganesan Parthiban (2010) Recent Advances in Novel Drug Delivery Systems. International Journal of Research in Ayurveda and Pharmacy 1(2): 316-326.
- VJ Mohanraj, Y Chen (2006) Nanoparticles a Review. Tropical Journal of Pharmaceutical Research 5(1): 561-573.
- Majeti N Ravi Kumar (2000) Nano and microparticles as controlled drug delivery device. Journal of pharma and pharmaceutical sciences 3(2): 234-258.
- Martins Ochubiojo Emeje, Ifeoma Chinwude Obidike, Ekaete Ibanga Akpabio, Sabinus Ifianyi Ofoefule (2012) Nanotechnology in Drug Delivery. Recent Advances in Novel Drug Carrier Systems (1): 69- 106.
- Frank Alexis, Eric Pridgon, Linda K Molnar, Omid C Farokhzad (2008) Factors Affecting the Clearance and Bio distribution of Polymeric Nanoparticles. Molecular Pharmaceutics 5(4): 505-515.
- Anne des Rieux, Virginie Fievez, Marie Garinot, Yves-Jacques Schneider, Véronique Préat (2006) Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. Journal of controlled drug release 116: 1-27.
- NK Jain (2001) Advances in controlled and novel Drug Delivery, CBS publisher & Distributers. (1st Edn) pp. 408.
- Dipti Desai, Dushyant Shah (2014) Implication of Nanoparticles for Controlled Drug Delivery System. IJPSR 3(6): 1125-1133.
- Khar RK, Vyas SP (2002) Nanoparticles in targeted and controlled drug delivery novel carrier systems. CBS publishers and distributors, New Delhi, India, pp. 331-385.
- Schmid G (2004) Nanoparticles: from theory to applications. Weinheim, Germany: Wiley-VCH Publishers.
- Geckeler KE, Rosenberg E (2006) Functional nanomaterials. American Scientific Publishers, Valencia, USA.
- Jilsha G, Vidya Viswanad (2013) Nanosponges: A Novel Approach of Drug Delivery System. International journal of pharmaceutical Sciences Review 19(2): 119-123.
- Azza Hassan A (2012) Formulation and evaluation of dorzolamide hydrochloride-loaded nanoparticles as controlled release drug delivery system. Asian Journal of Pharmaceutics 6(1): 67-73.
- M Bharathi, Sarat Chandra Prasad M, R Latha Eswari, S Wasim Rajad, Ravi Teja Allenab (2012) Preparation and in vitro & in vivo characterization of valsartan loaded eudragit nanoparticles. Der Pharmacia Sinica 3(5): 516-525.
- Tamizharsi S, Shukla A, Shivakumar T, Rathi V, Rathi JC (2009) Formulation and Evaluation of Lamivudine Loded Polymethaacrylic Acid Nanoparticles. International Journal of Pharmaceutical Technology and Research 1(3): 411-415.
- Anbarasan B, Venya Menon, S Ramaprabhu, Niranjana V A (2013) Optimization of the formulation and in-vitro evaluation of chloroquine loaded chitosan nanoparticles using ionic gelation method. Journal of Chemical and Pharmaceutical Sciences 6(2): 106-112.
- Paresh N Patel, LJ Patel, JK Patel (2011) Development and testing of novel temoxifen citrate loaded chitosan nanoparticles using ionic gelation method. Der Pharmacia Sinica 2(4): 17-25.
- Jaimin D patel, Sachin P Chauhan, AK Sethi (2012) Formulation & evaluation of methotrexate loaded nanoparticle. International Journal of Drug Discovery and Medical Research 1(2): 513-525.
- G Poovi, UM Dhana laxmi, N Narayana, P Neelakanta Reddy (2011) Preparation and Characterization of Repaglinide loaded Chitosan nanoparticles. Journal of Nanoscience and nanotechnology 1(1): 12-24
- Krishna Sailaja A, Amareshwar (2011) Preparation of chitosan coated nanoparticles by emulsion polymerization technique. Asian J Pharm Clin Res 4(11): 73-74.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...